doi: http://dx.doi.org/10.7705/biomedica.v35i3.2589
ARTÍCULO ORIGINAL
Author´s contributions:
Zinnia J. Molina and Lucio Galaviz designed the study, prepared the samples, and conducted the epidemiological study.
Daniel P. Molina and Roberto Mercado entomologically classified the captured insects, performed the histopathology, analyzed and interpreted the data, and drafted and edited the manuscript.
Recibido: 12/11/14; aceptado: 22/04/15
Introduction: Four species of triatomines have been reported in Nuevo León, northeast (NE) México, but Triatoma gerstaeckeri has only been recorded from a peridomestic dwelling.
Objectives: To assess the natural infection index (NII) of Trypanosoma cruzi in triatomines and the infestation index (II) of T. gerstaeckeri collected in a suburban locality, and to collect histopathological data to understand tissue tropism of the regional T. cruzi strain (strain NE) obtained from the vectors collected after an experimental inoculation in Mus musculus .
Materials and methods: Triatomines were collected from 85 houses and peridomiciles in Allende, Nuevo León. Stool samples were obtained to determine the T. cruzi NII and were used in an experimental mice infection.
Results: A total of 118 T . gerstaeckeri were captured, and 46 (adults and nymphs) were collected inside the same house (II=1.17%). Thirty-seven reduvids were infected with T. cruzi (NII=31.3%). Tissue tropism of the T. cruzi NE strain was progressive in skeletal muscle, myocardial, and adipose tissues and was characterized by the presence of intracellular amastigotes and destruction of cardiac myocells.
Conclusions: The presence of naturally infected domiciliary vectors is an important risk factor for public health in the region considering that these vectors are the principal transmission mechanism of the parasite. The T. cruzi NE strain has similar virulence to that of other Mexican and Texan strains and caused chagasic infections in 11 of 12 mice.
Key words: Trypanosoma cruzi , Triatoma , pathology, virulence, Chagas disease.
doi: http://dx.doi.org/10.7705/biomedica.v35i3.2589
Triatoma gerstaeckeri (Hemiptera: Reduviidae) infectada con Trypanosoma cruzi en Nuevo León, México, y capacidad patógena de la cepa regional
Introducción. En Nuevo León, localizado en el noreste de México, existen cuatro especies de triatominos, de las cuales Triatoma gerstaeckeri ha sido la única reportada en peridomicilios.
Objetivos. Evaluar el índice de infección natural de Trypanosoma cruzi en los triatominos y el índice de infestación de T. gerstaeckeri en una localidad suburbana, y obtener datos histopatológicos para comprender el tropismo tisular de la cepa regional (cepa NE) de T. cruzi obtenida de los vectores recolectados después de la infección experimental en Mus musculus.
Materiales y métodos. La recolección de triatominos se llevó a cabo en 85 casas y peridomicilios de Allende, Nuevo León, México. Se obtuvieron muestras de las deyecciones para conocer el índice de infección natural por T. cruzi y, con estas, se hicieron inoculaciones experimentales en ratones.
Resultados. Se capturaron 118 especímenes de T. gerstaeckeri , 46 (adultos y ninfas) en el mismo domicilio (índice de infestación=1,17 %). Treinta y siete redúvidos estaban infectados con T. cruzi (índice de infección natural, 31,3). El tropismo tisular de la cepa NE de T. cruzi fue progresivo en músculo esquelético, miocardio y tejido adiposo, y se caracterizó por la presencia de amastigotes intracelulares con destrucción de células cardiacas.
Conclusiones. La presencia de vectores domiciliarios naturalmente infectados con T. cruzi , es un factor de riesgo importante para la salud pública de la región, considerando que este es el principal mecanismo de la transmisión del parásito y que la cepa NE de T. cruzi tiene una virulencia similar a la de otras cepas mexicanas y texanas, y causó infección chagásica en 11 de los 12 ratones inoculados.
Palabras clave: Trypanosoma cruzi , Triatoma, patología, virulencia, enfermedad de Chagas.
doi: http://dx.doi.org/10.7705/biomedica.v35i3.2589
American trypanosomiasis, or Chagas disease, is a major health problem in rural, urban, and suburban areas of the Americas (1). Few studies of Chagas disease have been conducted in north- eastern México, which was considered free of Trypanosoma cruzi infection until 2009, because no reports of human cases were recorded. We recently demonstrated a mean seropositivity of 2.8% (2), but we did not address whether transmission of T. cruzi could occur from intradomiciliary or peridomiciliary hematophagous vectors.
Thirty-four triatomine species have been reported in México (3); however, only four species exist in the NE region of the country (4), where Triatoma gerstaeckeri has been collected most frequently from sylvatic habitats in association with the woodrat, Neotoma micropus (5). Few reports have shown infestations in domiciliary or peridomestic dwellings. Furthermore, no reports have focused on the tissue tropism of T. cruzi strains from NE Mexico and their public health implications.
Thus, we assessed the infestation index (II) and natural infection index (NII) of T. cruzi in T. gerstaeckeri collected in a suburban locality and gathered histopathological data to understand the tissue tropism and clinical importance of this T. cruzi strain (hereafter the T. cruzi NE strain). We also used the vectors collected for an experimental inoculation of Mus musculus .
Materials and methods
Study area
Sampling was carried out in the municipality of Allende, Nuevo León State, México, which shares its northern border with Texas, USA, and is located at 25°13´ N and 100°08´ W (figure 1) at the foot of the Sierra Madre Oriental, with valleys and hills ranging from 300 m above sea level in the northeastern region and up to 1,640 m above sea level in the southeastern region of the municipality. The mean annual temperature is 21.2°C, with a maximum of 40.1°C and a minimum of -2.4°C. The mean annual precipitation in the municipality is 1,055 mm (according to the Instituto Nacional de Estadística y Geografía) (6).

Specimen collection, infestation index, and natural infection index
This study was conducted from February to August, 2013. The sample size was determined using an estimated vector prevalence of 2.3% reported recently by our group (7). Eighty-five domiciles were visited of 7,500 houses in the municipality, with an absolute precision of 1.96, confidence level of 95%, and E =1.96, which exceeded the calculated sample size of 35 houses. Triatomines were collected in intradomiciles and peridomiciles according to a systematic random-stratified model. Inclusion criteria included a community socio-economic index, which was developed using household characteristics (wall, roof, and floor materials). The domiciles were assigned numerical values of 0, 1, or 2, representing three economic strata. Two to 10 housing blocks were selected for sampling randomly from maps (6), according to the density of each population. The selection procedure for the houses to be sampled involved enumerating each house in the block and selecting five houses on each side (6). Consent of the householder was the final inclusion criterion. If consent was refused, the house was replaced with a house nearby (8,9).
The timed manual collection consisted of searching for bugs inside dwellings and peridomestic habitats. The intradomiciliary searches focused on objects where triatomines could hide, and cracks and crevices in the walls, floors, and roofs, as well as areas around tables and chairs in the kitchen and bedrooms, were checked for 30 min by two searchers per house (10). Peridomestic annexes, including hen houses, corrals, storerooms, and wood-piles, were searched by two searchers for 30 min (10). Light traps were set up for collections in peridomestic and sylvatic areas using UV light-trap devices and plastic containers (model GT-200; Gardned Products, Horicon, WI, USA). The surveys were performed from 18:00 to 6:00 h (11,12).
All captured specimens were placed in glass containers covered with nylon mesh, and vertically folded cardboard was placed inside as a substrate. Each container was labeled according to the collection site and transported to the laboratory for identification (3,4). Stool samples were obtained by abdominal compression to determine the T. cruzi NII and to perform the experimental infection. A fresh stool aliquot was examined using an optical microscope (model DM 750; Leica, Ballerup, Denmark) to observe the trypomastigote forms in vivo (5).
Experimental infection
An experimental infection was performed using 5-week-old NHI male mice ( M. musculus ). Two groups (A and B) of six mice each were kept under standard conditions. Each mouse was injected intraperitoneally with 1 x 10 5 metacyclic trypomastigotes of the T. cruzi NE strain. Parasitemia was evaluated every 3 days using a hemocytometer (Cat. 1490; Hausser Scientific Co., Horsham, PA, USA), and the values are expressed as parasites/ ml (13). In addition, one group of 10 mice was monitored daily for survival over 100 days. Infected mice were euthanized by exsanguination under anesthesia on day 20-55 post-infection (dpi). Tissue samples were processed for histopathology and stained with hematoxylin and eosin.
The data of the arithmetic means for the pre-patent period, parasitemia values, dpi, tissue lesions, predominant tropism, and percent survival between the two groups were analyzed using Student´s t-test and the variance to detect differences using SPSS ver. 17 software (SPSS, Inc., Chicago, IL, USA).
Ethics statement
This study protocol was in strict accordance with the Universidad Autónoma de Nuevo León ethics committee recommendations. All mice were maintained under pathogen-free conditions in the animal facility at the Facultad de Ciencias Biológicas . The animals had free access to food and water and were handled in compliance with European standards. The mice were euthanized in a CO 2 chamber, and all efforts were made to minimize suffering.
Results
Natural infection and infestation indices
A total of 1 18 T . gerstaeckeri reduvids were collected (19 stage III nymphs, 36 stage IV nymphs, and 63 adults). Seventy-two reduvids were collected in peridomiciles. Forty-six triatomines (adult and nymphs) were collected from inside the same house (table 1), with an II of 1.17%.
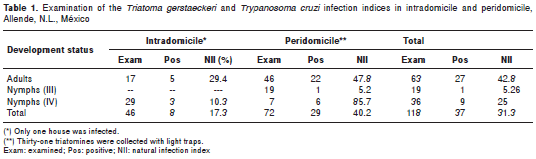
Thirty-seven (37/118, 31.3%) houses were infected with T. cruzi. The greatest II (40.2%) was in a peridomicile where 72 triatomines were collected. Five adult triatomines (5/17, 29.4%) and three nymphs (3/29, 10.3%) were infected with T. cruzi (NII=17.3%) inside the same house. The other entomological indices could not be calculated because it was impossible to determine whether the light trap-captured triatomines were sylvatic or peridomiciliary.
Trypanosoma cruzi NE strain pathogenicity and tissue tropism
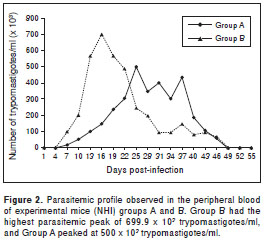
The experimental infection revealed a mean pre-patent period of 7 dpi. The highest and earliest parasitemia peak in mice from groups A and B was observed between 16 and 25 dpi (p=0.08) (figure 2). The mice showed hindquarter atrophy during the acute phase and paralysis on 28-29 dpi for the next 5 days, during which the parasitemia persisted in both groups. Peripheral-blood parasite counts peaked at 5-7 x 10 5 trypomastigotes per ml in both groups. No parasites were observed in mouse 5B. The differences in tissue lesions, predominant tropism, and the percent survival between the two experimental groups were not significant ( p=0.669), and the variances were similar.
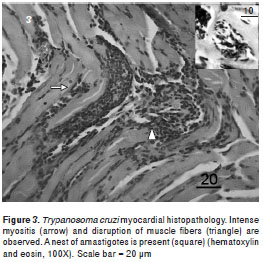
The anatomopathological damage caused by T. cruzi did not differ between the groups. Histopa- thological analyses of mice infected with T. cruzi strain NE showed mononuclear infiltrated cells and progressive myositis in skeletal muscle, myocardial, and adipose tissues, which were observed in mice euthanized 20 dpi. Intracellular amastigote nests and destroyed myocells accompanied by perivasculitis and adipose tissue degeneration were observed, which became more intense 30 dpi. Additionally, intense myocarditis was characterized by interstitial inflammation with predominant mononuclear infiltrates due to myocardial invasion.
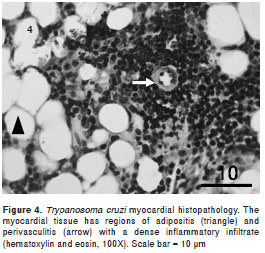
The intramyocytic presence of amastigote nests, interstitial inflammation, disorganized muscle fibers (figure 3), and an inflammatory infiltrate of perivascular adipose tissue (figure 4) were evident in skeletal muscle 40-50 dpi. The smooth muscle layer in the digestive tract showed a few nests of amastigote forms with an intense inflammatory reaction. Amastigote nests were also observed in Kupffer cells. No mortality was observed during the survival experiment.
Discussion
Natural infection and infestation indices
The distribution of T. gerstaeckeri extends from central México through Texas and New Mexico, USA, (14) and is commonly associated with pack rat ( Neotoma spp.) burrows as a sylvatic species (5,15). Some reports have described T. gerstaeckeri as a human and livestock pest species but the adults are frequent invaders of rural houses in southwestern USA, which agrees with our findings.
This is the first report of T. gerstaeckeri nymphs and adults inside a dwelling in Nuevo León, México, but reports of humans being bitten are common in southwestern USA (15). Another study reported possible colonization based on observations of a large number of immature stage bugs in a Cameron (Texas, USA) residence where 31 triatomines were collected after three pet dogs died from Chagas cardiomyopathy (24 contained T. cruzi ) (15 ). These findings highlight the importance of epidemiological studies of T. gerstaeckeri in this geographical region .
Our results are considered an additional case, as we collected 46 triatomines inside a single home, and three employees of the field house were later confirmed to be anti- T. cruzi antibody seropositive. A 1992 survey of some villages in the municipality of General Terán, N.L., which is 90 km from our study site, revealed that 181 of 192 (94%) T. gerstaeckeri specimens were found in or around houses , with an II of 24% and an NII of 28% (16). Furthermore, this particular species was frequently associated with human dwellings in 2009, when 113 (63%) of 180 T. gerstaeckeri specimens collected were found in domestic settings.
Indeed, infected T. gerstaeckeri specimens were recovered recently from the southern Texas residence of a child with acute Chagas disease (17). Similarly, the distribution of T. gerstaeckeri was extended through the central region of México in 2008 to San Luis Potosí, Veracruz, Querétaro (18), and Hidalgo, as well as inside human dwellings (19). The presence of T. gerstaeckeri nymphs inside houses suggests that this triatomine is adapting to houses and that colonization has occurred, based on the presence of nymphs in Texas, USA (14,15,17) and Querétaro (18). Thus, vectorial transmission has increased, and thus the risk of Chagas disease.
Tissue tropism and pathogenicity
Similar low infection peaks (~1-1.5 x 10 6 ) have been observed in Central American (11,20) and other Mexican strains (21). We elucidated the myotropic behavior of the T. cruzi NE strain by examining invasive capacity into the muscle and adipose tissue of the myocardium. The infective capacity of a Venezuelan isolate of T. cruzi was demonstrated in fatty connective tissue and skeletal and cardiac muscle (13); however, future studies are needed to determine the phylogenetic lineage of the T. cruzi NE strain (22).
The predominance of T. cruzi and its pathogenicity, histopathological analyses , and strain virulence have been described in eight of the 32 Mexican states, showing that T. cruzi exhibits considerable intraspecific diversity, as exemplified by differences in morphology of blood forms, virulence, and pathogenicity (23). Mexican strains that are preferentially cardiomyotropic have been reported in Jalisco, Guerrero , and Zacatecas; the Tetitlán strain infects nerves, stomach, brain, and striated muscle, limiting mice survival to 26-30 dpi, whereas the Tepechitlán strain (Zacatecas) causes death in 100% of mice 15-20 dpi (24). The Querétaro strain results in 100% mortality, whereas no mortality was observed in mice infected with the Ninoa strain (25). We report a similar lack of mortality in our survival experiment. Furthermore, both strains produce extended lymphocytic infiltrates in cardiac tissue (25).
The histotropic behavior and pathogenicity of the T. cruzi strains differ between areas in México (19), but the most frequently reported tissues infected with T. cruzi are the visceral organs, peritoneum, and central nervous system (24,26). The NE strain of T. cruzi established itself in the experimental host and caused the development of a chagasic infection in 11 of 12 experimental mice. The third experimental group of mice showed parasitemia peaks that reached 699.9 x 10 3 parasites/ml, which could be the lowest parasitemia level among the strains that have been analyzed in México (21,25). A T. cruzi strain established in southeastern USA was analyzed by Hall in 2010 (13), and their tropism and low virulence results agree with our observations from the third experimental group.
These results contrast with the tropism and infectivity of some populations of T. cruzi strains widely documented in Central and South America (27,28), which have higher public health significance because they can cause "silent" disease. Our findings suggest that the presence of a vector naturally infected with T. cruzi is an important public health risk factor for the region, considering that it is the principal transmission mechanism. Therefore, programs should be developed to interrupt vectorial transmission. In addition, it is necessary to promote recognition and identification of the vector in the community because Chagas disease is currently viewed as an exotic illness by public health personnel (29).
We gratefully acknowledge the contributions of the chemistry-bacteriology-parasitology students of the Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, for their assistance with the field collections and laboratory work. In addition, special thanks go to Biol. Lidia Baylón (Departamento de Infectómica y Patogénesis Molecular, Centro de Investigación y de Estudios Avanzados, México), who kindly helped us culture the T. cruzi NL strain.
None declared.
This study was supported by grant N° UANL-CA-278 from the PROMEP/103.5/11/1047 P/CA to LGS and partially supported by PAICYT-UANL CN835-11.
Corresponding author: Zinnia J. Molina-Garza, Departamento de Zoología de Invertebrados, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Unidad B, Avenida Universidad, Ciudad Universitaria, San Nicolás de los Garza, Nuevo León, CP 66451, México Telephone/fax: +52-81-8352-4425 molinazinnia@hotmail.com
1. Sánchez MC, Barnabé C, Guégan JF, Tibayrenc M, Velázquez M, Martínez J. High prevalence anti- Trypanosoma cruzi antibodies, among blood donors in the State of Puebla, a non-endemic area of México. Mem Inst Oswaldo Cruz. 2002;97:947-52. http://dx.doi.org/10.1590/S0074-02762002000700004
2. Galaviz L, Molina DP, González MA, Mercado R, Rosales JL, Molina ZJ. Update on seroprevalence of anti- Trypanosoma cruzi antibodies among blood donors in Northeast México. Am J Trop Med Hyg. 2009;81:404-6.
3. Salazar P, Rojas G, Cabrera M, Bucio I, Martínez J, Monroy M , et al . Revisión de 13 especies de la familia Triatominae (Hemiptera: Reduviidae) vectores de la enfer-medad de Chagas, en México. J Selva Andina Res Soc. 2010;1:57-80.
4. Cruz R, Pickering JM. Chagas disease in Mexico: An analysis of geographical distribution during the past 76 years - A review. Mem Inst Oswaldo Cruz. 2006;101:345-54. http://dx.doi.org/10.1590/S0074-02762006000400001
5. Molina ZJ, Rosales JL, Galaviz L, Molina D . Prevalencia de Trypanosoma cruzi en triatominos silvestres de Nuevo León, México. Salud Pública Mex. 2007;49:37-44.
6. Instituto Nacional de Estadística, Geografía e Informática. Cuaderno Estadístico Municipal. Fecha de consulta: 22 de septiembre de 2014. Disponible en: http://www.inegi.org.mx/est/contenidos/espanol/sistemas/cem03/estatal/nln/m004/index.htm.
7. Molina ZJ, Rosales JL, Mercado R, Molina DP, Gómez R, Galaviz L. Association of Trypanosoma cruzi infection with risk factors and electrocardiographic abnormalities in northeast México. BMC Infec Dis. 2014;14:117. http://dx.doi.org/10.1186/1471-2334-14-117
8. Rojas ME, Várquez P, Villarreal MF, Velandia C, Vergara L, Morán YH. Estudio seroepidemiológico y entomológico sobre la enfermedad de Chagas en un área infestada por Triatoma maculata (Erichson 1848) en el centro-occidente de Venezuela. Cad Saúde Pública. 2008;24:2323-33. http://dx.doi.org/10.1590/S0102-311X2008001000013
9. Salazar JJ, Gallego LM, Suárez B, Heredia H, Hernández T, Naranjo M. Seroepidemiological study of Chagas disease in the community of Copey-El Guayabillo, State of Carabobo, Venezuela. Rev Cubana Med Trop . 2014;66:34-47.
10. Mejía AM, Agudelo-Uribe LA, Dib JC, Ortiz S, Solari A, Triana C. Genotyping of Trypanosoma cruzi in a hyper- endemic area of Colombia reveals an overlap among domestic and sylvatic cycles of Chagas disease. Parasit Vectors. 2014;7:108. http://dx.doi.org/10.1186/1756-3305-7-108
11. Angulo VM, Esteban L, Urbano P, Hincapié E, Núñez LA. Comparación de métodos para la captura de triatominos (Hemiptera: Reduviidae) en palmas Attalea butyracea en los Llanos Orientales de Colombia. Biomédica. 2013;33:653-9. http://dx.doi.org/10.7705/biomedica.v33i4.835
12. Minoli SA, Lazzari CR. Take-off activity and orientation of triatomines (Heteroptera: Reduviidae) in relation to the presence of artificial lights. Acta Trop. 2006;97:324-30.
13. Hall CA, Pierce EM, Wimsatt AN, Hobby T, Meers JB. Virulence and vertical transmission of two genotypically and geographically diverse isolates of Trypanosoma cruzi in mice. J Parasitol. 2010;96:371-6. http://dx.doi.org/10.1645/GE-2296.1
14. Sarkar S, Strutz SE, Frank DM, Rivaldi CL, Sissel B, Sánchez-Cordero V. Chagas disease risk in Texas. PLoS Negl Trop Dis. 2010:4:e836 . http://dx.doi.org/10.1371/journal.pntd.0000836
15. Beard CH, Pye G, Steurer FJ, Rodríguez R, Campman R, Peterson AT, et al . Chagas disease in a domestic transmission cycle in Southern Texas, USA. Emerg Infect Dis. 2003;9:103-5. http://dx.doi.org/10.3201/eid0901.020217
16. Martínez JA, Galaviz L, Lara C, Trujillo C. Distribución de los triatominos asociados al domicilio humano en el municipio de General Terán, Nuevo León, México. Southwestern Entomol. 1992;17:261-5.
17. Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41-50. http://dx.doi.org/10.1089/vbz.2008.0026
18. Villagrán ME, Marín C, Hurtado A, Sánchez M, de Diego JA. Natural infection and distribution of triatomines (Hemiptera: Reduviidae) in the state of Querétaro, México. Trans R Soc Trop Med Hyg. 2008;102:833-8. http://dx.doi.org/10.1016/j.trstmh.2008.05.005
19. Vidal V, Ibáñez S, Martínez C. Infección natural de chinches Triatominae con Trypanosoma cruzi asociadas a la vivienda humana en México. Salud Pública Mex. 2000;42:496-503.
20. Calderón O, Chinchilla M, García F, Vargas M. Variaciones biológicas de Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) asociadas con la ingestión de diferentes tipos de sangre por el vector Triatoma dimidiata (Hemiptera: Reduviidae). Parasitol Latinoam. 2003;58:3-10. http://dx. doi.org/10.4067/S0717-77122003000100001
21. Sánchez MC, Bernabé C, Tibayrenc M, Zavala J, Totolhua JL, Méndez J, et al. Trypanosoma cruzi strains isolated from human, vector, and animal reservoir in the same endemic region in México and typed as T. cruzi I, discrete typing unit 1 exhibit considerable biological diversity. Mem Inst Oswaldo Cruz. 2006;10:585-90. http://dx.doi.org/10.1590/S0074-02762006000600002
22. Liarte DB, Murta SM, Steindel M, Romanha AJ. Trypanosoma cruzi : Multiplex PCR to detect and classify strains according to groups I and II. Exp. Parasitol. 2009;123: 283-91. http://dx.doi.org/10.1016/j.exppara.2008.12.005
23. Bosseno MF, Barnabé C, Magallón E, Lozano F, Ramsey J, Espinoza B, et al. Predominance of Trypanosoma cruzi Lineage I in México. J Clin Microbiol. 2002;40:627-632. http://dx.doi.org/10.1128/JCM.40.2.627-632.2002
24. León FE, Prada DG, Bayona J, Valderrama V, García I, León ME, et al . Neurotripanosomiasis americana: aspectos clínicos de un problema básico. Biomédica. 2003;23:462- 75. http://dx.doi.org/10.7705/biomedica.v23i4.1240
25. Espinosa B, Rico T, Sosa S, Oaxaca E, Vizcaíno A, Caballero ML. Mexican Trypanosoma cruzi TCI strains with different degrees of virulence induce diverse humoral and cellular immune responses in a murine experimental infection model. J Biomed Biotechnol. 2010;2010:890672. http://dx.doi.org/10.1155/2010/890672
26. Salazar PM, De Haro Al, Tay J, Bucio MI, Robert L. Estudio de la virulencia de cepas de Trypanosoma cruzi en el ratón blanco. Rev Mex Patol Clin. 1987;34:105-9.
27. Lauria L, Santana JM, Tavares FS, Teixeira AR. Diversity of T. cruzi stocks and clones derived from Chagas´ disease patients: I-Behavioral characterization in vitro. Rev Soc Bras Med Trop. 1997;30:187-90. http://dx.doi.org/10.1590/S0037-86821997000300003
28. Rodríguez HO, Guerrero NA, Fortes A, Santi-Roccab J, Gironès N, Fresno M. Trypanosoma cruzi strains cause different myocarditis patterns in infected mice. Acta Trop. 2014;139:5-66. http://dx.doi.org/10.1016/j.actatropica.2014.07.005
29. Cárdenas JD, Mazariego MA, Utrilla FJ, Monteón VM, Altúzar M. Anticuerpos anti- Trypanosoma cruzi en pacientes con cardiomiopatía dilatada. Rev Méd IMSS. 2003;41:111-4.