Biomédica 2017;37:303-7
doi: https://doi.org/10.7705/biomedica.v34i2.3189
CASE PRESENTATION
1 Servicio de Enfermedades Infecciosas, Clínica Cardio VID, Medellín, Colombia
2 Escuela de Ciencias de la Salud, Universidad Pontificia Bolivariana, Medellín, Colombia
3 Servicio de Patología, Laboratorio Clínico VID, Medellín, Colombia
4 Sección de Dermatología, Departamento de Medicina Interna, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Author’s contributions:
Victoria Dávila prepared the draft version of the manuscript.
All authors contributed in the collection of clinical and laboratory data, the conception and design of the manuscript, and they all approved its final version.
Received: 17/12/15; accepted: 20/10/16
We report the case of a 61 year-old male who underwent heart transplantation eight months before developing a systemic condition with central nervous system, lung, kidney, colonic, cutaneous, and hematologic involvement, found to be secondary to a systemic toxoplasmosis despite co-trimoxazole prophylaxis in a previous-to-transplant seronegative patient receiving a heart from a seropositive donor. A review of prophylactic options in our environment is discussed.
Key words Toxoplasmosis; heart transplantation; immune tolerance; seroconversion.
doi: https://doi.org/10.7705/biomedica.v34i2.3189
Toxoplasmosis diseminada en un paciente con trasplante de corazón a pesar de la profilaxis con trimetoprim-sulfametoxazol
Se reporta el caso de un paciente de sexo masculino, de 61 años de edad, quien ocho meses después de someterse a un trasplante de corazón presentó una enfermedad sistémica con compromiso del sistema nervioso central y del sistema inmunológico, así como de pulmón, riñón, colon y piel, y a quien finalmente se le diagnosticó toxoplasmosis diseminada, a pesar de haber recibido profilaxis con trimetoprim-sulfametoxazol, debido a que el órgano provenía de un donante positivo para toxoplasmosis siendo él un receptor negativo. Se discuten las opciones de profilaxis en nuestro medio.
Palabras clave: toxoplasmosis; trasplante de corazón; tolerancia inmunológica; seroconversión.
doi: https://doi.org/10.7705/biomedica.v34i2.3189
Toxoplasmosis is an infection caused by the protozoon Toxoplasma gondii. It is prevalent worldwide, affecting approximately one third of the world’s population (1). Seroprevalence increases with age and there is no gender difference.
Toxoplasmosis is transmitted via oral intake of food contaminated with the parasite’s cysts or oocysts. However, there are reports of transmission through transplanted organs and blood transfusions, as well as vertical transmission (2).
It evolves to a systemic disease, and only 10% of the affected population is symptomatic. The clinical course is usually benign, but it can be lethal, especially in immunosuppressed patients (3). Systemic clinical signs include generalized adenopathies, myocarditis, polymyositis, pneumonitis, encephalitis and chorioretinitis. Skin manifestations are rare, though usually present in immunosuppressed patients. Given the great variability in the appearance of cutaneous toxoplasmosis, histopathological studies are required for diagnosis.
Although direct observation of the parasite is possible in most tissue samples, diagnosis should be confirmed by immunohistochemistry and polymerase chain reaction (PCR) (4).
Medical therapy is indicated when there is active skin or ocular infection, or congenital disease, and in all immunosuppressed patients. Sulfadiazine in combination with pyrimethamine is the treatment of choice. Seronegative heart transplant patients should receive prophylaxis with pyrimethamine and sulfadiazine for the first three months followed by co-trimoxazole for the rest of their lives (5,6).
Case report
We report the case of a 61-year-old male patient with a past medical history of hypertension, dyslipidemia, hypothyroidism, heart failure with preserved ejection fraction and severe coronary artery disease, in whom surgical and percutaneous revascularization were performed. After surgery, the patient developed refractory angina despite optimal anti-ischemic medication which led to heart transplantation. Laboratory tests previous to transplant were discordant for T. gondii and human herpes viruses type I and II. The donor had positive immunoglobulin M (IgM), negative immunoglobulin G (IgG) for T. gondii, and positive IgG for herpes viruses (HV) type I and type II. The receptor was negative for both.
After eight months of transplant the patient was admitted with persistent diarrhea lasting 20 days, abdominal pain and malaise. The immunosuppression regimen included mycophenolate mofetil, 1 g every 12 hours, prednisolone, 5 mg per day, and cyclosporine, 150 mg every 12 hours. Prophylaxis with co-trimoxazole, 160/800 mg per day, was also given.
On physical examination the patient presented with tachycardia, normal blood pressure and respiratory rate, and no fever was documented. Vesicular lesions with clear content and erythematous base with dermatome distribution were seen in the right gluteus.
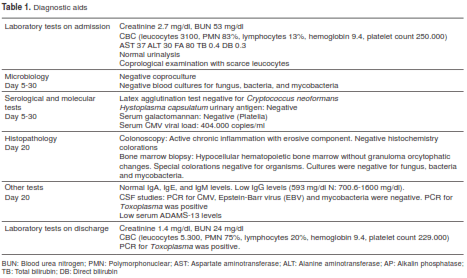
Initial laboratory tests showed anemia, leukopenia with lymphopenia, renal dysfunction and a positive Tzank test (table 1). Viral load for cytomegalovirus (CMV) was positive with 404.000 copies/ml; therapy with ganciclovir, 5 mg/kg every 12 hours, was started with initial improvement of the diarrhea.

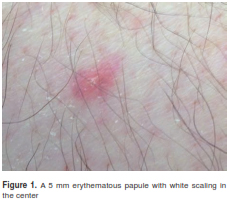
However, two weeks later the patient persisted with leukopenia, the anemia worsened requiring red blood cell (RBC) transfusion and he developed fever as high as 39.8o C. The only additional finding was four 5 mm to 1 cm erythematous, mildly painful lesions, some in legs and thighs with white scaling in the center, and a similar lesion in the upper right eyelid (figure 1). The diarrhea reappeared, renal dysfunction worsened, as well as the complete blood count (CBC), now with pancytopenia (anemia, leukopenia, lymphocytopenia, neutropenia and thrombocytopenia).
Empirical therapy with anidulafungin plus cefepime was started for febrile neutropenia. Toxoplasma antibody seroconversion was documented. Therapy with clindamycin plus pyrimethamine was started and the skin lesions were biopsied.
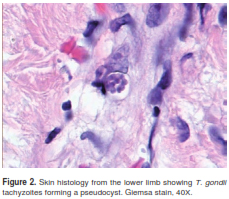
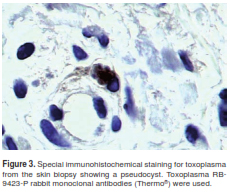
Histopathology of skin lesions was positive and immunohistochemistry for toxoplasmosis confirmed the presence of the parasite (figure 2 and figure 3). Molecular studies with blood and cerebrospinal fluid (CSF) PCR were also positive for Toxoplasma (7). Central nervous system magnetic resonance (MR) did not show focal lesions.
The initial course was unfavorable, the patient persisted with fever, headache, encephalopathy, oliguric renal failure, hepatitis, pancytopenia, metabolic acidosis and increased lactate dehydrogenase (LDH) up to 2.500 IU/L. Pulmonary patchy infiltrates were found. He was admitted to the intensive care unit for mechanical ventilation. Plasma exchange was started for suspected thrombotic thrombocytopenic purpura.
After a few days the patient ́s status improved, especially renal function and blood counts, but he persisted with hypogammaglobulinemia. CMV infection relapsed with poor response to ganciclovir. Gammaglobulin, 30 g intravenous (IV) weekly, was started with complete remission.
He was released after six weeks of therapy with clindamycin and pyrimethamine upon suspicion of co-trimoxazole resistance. Suppression with cotrimoxazole plus valganciclovir and gammaglobulin was maintained and the patient has been symptomfree for 28 months.
Discussion
This is a heart transplant patient who developed systemic toxoplasmosis with central nervous system, pulmonary, renal, colonic, cutaneous, and hematologic compromise secondary to disseminated toxoplasmosis. Toxoplasma gondii infection after heart transplant is often a severe disease. The clinical presentation ranges from chorioretinitis to disseminated infection (pulmonary, myocardial, hepatic and CNS infection) (8).
Toxoplasmosis in immunocompromised patients is usually secondary to a reactivation of latent infection from the donor (9). In this case, previous to transplantation the donor ́s toxoplasmosis serology was discordant with the recipient ́s. Toxoplasma cysts may persist in the donor’s myocardium, thus, hosts are at higher risk of developing the disease. With immunosuppression, the bradyzoites become tachyzoites, quickly proliferating and consequently destroying cells.
Before the use of prophylaxis with co-trimoxazole there was a higher risk of disseminated toxoplasmosis (10). Studies of seronegative heart recipients have found that the risk of T. gondii- associated clinical disease ranges from 0 to 82% if patients receive organs from seropositive donors without antimicrobial prophylaxis (6,8).
Seronegative heart transplant patients should receive prophylaxis. Effective regimens include one single-strength co-trimoxazole tablet daily, one double-strength three times a week, 1,500 mg atovaquone daily or pyrimethamine 25 mg/day. This patient had received prophylaxis with co- trimoxazole. Results from observational studies have shown that this drug is effective in preventing toxoplasmosis, even in cardiac recipients (6,11).
The patient may have developed severe disease despite co-trimoxazole prophylaxis due to resistance to sulfonamide (12,13). In this sense, more-virulent Toxoplasma strains have been found in Colombia and transmission of toxoplasmosis by renal transplant was reported in two local cases (14,15). This suggests that the risk could be higher in tropical zones of the Americas where a high genetic diversity of Toxoplasma has been found.
Co-trimoxazole is provided as a 1/5 ratio combination of trimethoprim and sulfamethoxazole. This ratio was chosen in order to provide synergistic ratio against bacteria, but due to the poor efficacy of trimethoprim against T. gondii trophozoites (100 times lower than pyrimethamine), the optimal ratio of the two compounds against this infection is probably closer to 1/1 (16). High doses of co- trimoxazole are needed to cure experimental acute infection in mice, and relapses are observed after cessation of therapy, because it is ineffective on the cyst form of the parasite. However, it has proved remarkably efficient in the treatment and prophylaxis of toxoplasmosis in transplant patients with AIDS (11).
Considering that a high genetic diversity, gene polymorphisms in the dihydrofolate reductase and dihydropteroate synthase genes, more-virulent strains of T. gondii, and several transplanted patients with disseminated toxoplasmosis have been reported in Colombia, the recommended doses of co-trimoxazole could be insufficient, or another agent (e.g., dapsone plus pentamidine or pyrimethamine and sulfadiazine) could be necessary to prevent toxoplasmosis in high risk transplant patients.
It is also important to point out that this patient developed symptoms after eight months of transplantation. Previous reports have described how heart transplant patients receiving the organ from seropositive donors usually develop infections four to twelve weeks after transplantation. According to previous reports, the organs most commonly affected by systemic toxoplasmosis in immunosuppressed patients are the lungs and the central nervous system (17,18).
To our knowledge, this is the first heart transplant patient reported in the literature presenting toxoplasmosis with cutaneous manifestations. Such cutaneous involvement is quite rare, and only a few cases have been reported in immunocompromised patients with great variability in the appearance of skin lesions. Generalized rash, erythema multiform- like lesions, nodular lesions and vesicular varicella- like lesions have been described (19-21). Skin biopsies confirmed the presence of the parasite, but histopathological findings are variable. The involvement of the colon is also unique in this patient.
We can conclude, then, that diagnosing toxoplasmosis in immunosuppressed patients is challenging, as any organ may be affected. Thus, toxoplasmosis should always be suspected as a differential diagnosis in patients with systemic symptoms. Patients must be studied thoroughly, using all possible diagnostic aids to rule out the disease, including serology, histology, special immunohistochemical staining and PCR (22-23). Co-trimoxazole prophylaxis does not exclude the diagnosis andresistance studies are necessary.
The authors declare no conflict of interests.
No funding was received.
Corresponding author: Gustavo E. Roncancio, Calle 78N N° 75-21, Medellín, Colombia Teléfono: (574) 445 4000, extensión 4314; fax: (574) 441 7837 groncancio@gmail.com1. Galván-Ramírez M de L, Troyo R, Román S, Calvillo- Sánchez C, Bernal-Redondo R. A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasit Vectors. 2012;5:271. https://doi.org/10.1186/1756-3305-5-271
2. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264-96. https://doi.org/10.1128/CMR.05013-11.
3. Lupi O, Bartlett BL, Haugen RN, Dy LC, Sethi A, Klaus SN, et al. Tropical dermatology: Tropical diseases caused by protozoa. J Am Acad Dermatol. 2009;60:897-925. https://doi.org/10.1016/j.jaad.2009.03.004
4. Zimmermann S, Hadaschik E, Dalpke A, Hassel JC, Ajzenberg D, Tenner-Racz K, et al. Varicella-like cutaneous toxoplasmosis in a patient with aplastic anemia. J Clin Microbiol. 2013;51:1341-4. https://doi.org/10.1128/JCM.02851-12
5. Wreghitt TG, Gray JJ, Pavel P, Balfour A, Fabbri A, Sharples LD, et al. Efficacy of pyrimethamine for the prevention of donor-acquired Toxoplasma gondii infection in heart and heart-lung transplant patients. Transpl Int. 1992;5:197-200. https://doi.org/10.1111/j.1432-2277.1992.tb01745.x
6. Fernández-Sabé N, Cervera C, Fariñas MC, Bodro M, Muñoz P, Gurguí M, et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: A matched case-control study. Clin Infect Dis.2012;54:355-61. https://doi.org/10.1093/cid/cir806
7. Patrat-Delon S, Gangneux JP, Lavoué S, Lelong B, Guiguen C, le Tulzo Y, et al. Correlation of parasite load determined by quantitative PCR to clinical outcome in a heart transplant patient with disseminated toxoplasmosis. J Clin Microbiol. 2010;48:2541-5. https://doi.org/10.1128/JCM.0025210
8. Luft BJ, Naot Y, Araujo FG, Stinson EB, Remington JS. Primary and reactivated toxoplasma infection in patients with cardiac transplants. Clinical spectrum and problems in diagnosis in a defined population. Ann Intern Med. 1983;99:27-31. https://doi.org/10.7326/0003-4819-99-1-27
9. Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39:895-901. https://doi.org/10.1016/j.ijpara.2009.02.004
10. Muñoz P, Valerio M, Eworo A, Bouza E. Parasitic infections in solid-organ transplant recipients. Curr Opin Organ Transplant. 2011;16:565-75. https://doi.org/10.1097/MOT.0b013e32834cdbb0
11. Orr KE, Gould FK, Short G, Dark JH, Hilton CJ, Corris PA, et al. Outcome of Toxoplasma gondii mismatches in heart transplant recipients over a period of 8 years. J Infect. 1994;29:249-53. https://doi.org/10.1016/S0163-4453(94)91082-0
12. Pfefferkorn ER, Borotz SE, Nothnagel RF. Toxoplasma gondii: Characterization of a mutant resistant to sulfonamides. Exp Parasitol. 1992;74:261-70. https://doi.org/10.1016/0014-4894(92)90149-5
13. Cortés LJ, Duque S, López MC, Moncada D, Molina D, Gómez-Marín JE, et al. Gene polymorphisms in the dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) genes and structural modelling of the dhps gene in Colombian isolates of Toxoplasma gondii. Biomédica. 2014; 34:556-66. https://doi.org/10.7705/biomedica.v34i4.2132
14. Mejía G, Leiderman E, Builes M, Henao J, Arbeláez M, Arango JL, et al. Transmission of toxoplasmosis by renal transplant. Am J Kidney Dis. 1983;2:615-7.
15. Álvarez C, de la Torre A, Vargas M, Herrera C, Uribe- Huertas LD, Lora F, et al. Striking divergence inToxoplasma ROP16 nucleotide sequences from human and meat samples. J Infect Dis. 2015;211:2006-13. https://doi.org/10.1093/infdis/jiu833
16. Brun-Pascaud M, Chau F, Garry L, Farinotti R, Derouin F, Girard PM. Altered trimethoprim-sulfamethoxazole ratios for prophylaxis and treatment of Toxoplasma gondii and Pneumocystis carinii dual infections in rat model. J AcquImm Defic Syndr Hum Retrovir. 1996;13:201-7. https://doi.org/10.1097/00042560-199611010-00001
17. Hermanns B, Brunn A, Schwarz ER, Sachweh JS, Seipelt I, Schröder JM, et al. Fulminant toxoplasmosis in a heart transplant recipient. Pathol Res Pract. 2001;197:211-5. https://doi.org/10.1078/0344-0338-00036
18. Chandrasekar PH, Momin F. Disseminated toxoplasmosis in marrow recipients: A report of three cases and a review of the literature. Bone Marrow Transplant. 1997;19:685-9.
19. Amir G, Salant H, Resnick IB, Karplus R. Cutaneous toxoplasmosis after bone marrow transplantation with molecular confirmation. J Am Acad Dermatol. 2008;59: 781-4. https://doi.org/10.1016/j.jaad.2008.07.014
20. Lee SA, Diwan AH, Cohn M, Champlin R, Safdar A. Cutaneous toxoplasmosis: A case of confounding diagnosis. Bone Marrow Transplant. 2005;36:465-6. https://doi.org/10.1038/sj.bmt.1705079
21. Zimmermann S, Hadaschik E, Dalpke A, Hassel JC, Ajzenberg D, Tenner-Racz K, et al. Varicella-like cutaneous toxoplasmosis in a patient with aplastic anemia. J Clin Microbiol. 2013;51:1341-4. https://doi.org/10.1128/JCM.02851-12
22. Holliman R, Johnson J, Savva D, Cary N, Wreghitt T. Diagnosis of toxoplasma infection in cardiac transplant recipients using the polymerase chain reaction. J Clin Pathol. 1992;45:931-2.
23. Costa JM, Pautas C, Ernault P, Foulet F, Cordonnier C, Bretagne S. Real-time PCR for diagnosis and follow-up of toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J Clin Microbiol. 2000;38:2929-32.