Biomédica 2017;37:164-74
doi: http://dx.doi.org/10.7705/biomedica.v37i2.3276ORIGINAL ARTICLE
1 Instituto Nacional de Enfermedades Respiratorias “Emilio Coni”, ANLIS “C.G. Malbrán”, Santa Fe, Argentina
2 Dirección de Bioquímica de la Municipalidad de Rosario, Rosario, Argentina
3 Hospital San Roque, San Salvador de Jujuy, Argentina
4 Laboratorio Central de Salud Pública, Resistencia, Argentina
5 Hospital “Dr. Francisco Javier Muñiz”, Ciudad Autónoma de Buenos Aires, Argentina
6 Hospital Interzonal General de Agudos “Evita”, Lanús, Argentina
7 Hospital Rawson, Córdoba, Argentina
Author’s contributions:
María Imaz: Conception and design of the study, analysis and interpretation of data and writing of the manuscript
Sonia Allassia, Mónica Aranibar, Alba Gunia, Susana Poggi, Ana Togneri and Lidia Wolff: Diagnostic tests, data collection, analysis and interpretation
Group of Implementation of Fluorescence Microscopy: Diagnostic tests, data collection
All authors participated in the review and approval of the final version of the manuscript.
Received: 28/03/16; accepted: 17/07/16
Introduction: Light-emitting diode fluorescence microscopy (LED-FM) has been endorsed by the World Health Organization (WHO) for tuberculosis diagnosis, but its accuracy in HIV-infected patients remains controversial, and only some few studies have explored procedural factors that may affect its performance.
Objective: To evaluate the performance of LED-FM for tuberculosis diagnosis in patients with and without HIV infection using a newer, less expensive LED lamp.
Materials and methods: We compared the performance of LED-FM and Ziehl-Neelsen (ZN) microscopy on respiratory specimen smears from tuberculosis (TB) suspects and patients on treatment examined by different technicians blinded for HIV-status and for the result of the comparative test. We analyzed the effect of concentrating specimens prior to microscopy using different examination schemes and user-appraisal of the LED device.
Results: Of the 6,968 diagnostic specimens collected, 869 (12.5%) had positive Mycobacterium tuberculosis cultures. LED-FM was 11.4% more sensitive than ZN (p<0.01). Among HIV-positive TB patients, sensitivity differences between LED-FM and ZN (20.6%) doubled the figure obtained in HIV- negative patients or in those with unknown HIV status (9.3%). After stratifying by direct and concentrated slides, the superiority of LED-FM remained. High specificity values were obtained both with LED-FM (99.9%) and ZN (99.9%).The second reading of a sample of slides showed a significantly higher positive detection yield using 200x magnification (49.4 %) than 400x magnification (33.8%) (p<0.05). The LED- device had a very good acceptance among the technicians.
Conclusion: LED-FM better performance compared with ZN in HIV-infected patients and user-appraisal support the rapid roll-out of LED-FM. Screening at 200x magnification was essential to achieve LED- FM increased sensitivity.
Key words: Tuberculosis; fluorescence; microscopy; diagnosis; sputum; acid-fast bacilli. doi: http://dx.doi.org/10.7705/biomedica.v37i2.3276
Rendimiento de la microscopía de fluorescencia LED para la detección de bacilos ácido-alcohol resistentes en muestras respiratorias en laboratorios periféricos de Argentina
Introducción. La microscopía de fluorescencia con lámpara LED (MF-LED) ha sido recomendada por la Organización Mundial de la Salud (OMS) para el diagnóstico de la tuberculosis, pero su precisión en pacientes con HIV continúa siendo controversial y en pocos estudios se han explorado los factores metodológicos que pueden afectar su utilidad.
Objetivo. Evaluar el rendimiento de la MF-LED en el diagnóstico de la tuberculosis en pacientes con HIV y sin él mediante un novedoso dispositivo LED.
Materiales y métodos. Se comparó el rendimiento de la MF-LED y la microscopía en frotis de muestras respiratorias con tinción de Ziehl-Neelsen (M-ZN) examinados por técnicos cegados en cuanto al estado de HIV y el resultado de la prueba comparativa. Se analizó el efecto de concentrar muestras antes de la microscopía, usar diferentes esquemas de observación y la valoración con el dispositivo LED.
Resultados. De las 6.968 muestras recolectadas, 869 (12,5 %) resultaron con cultivo positivo para Mycobacterium tuberculosis. La MF-LED fue 11,4 % más sensible que la M-ZN (p<0,01). Entre los pacientes con tuberculosis positivos para HIV, la diferencia de sensibilidad entre la MF-LED y la M-ZN (20,6 %) duplicó la cifra obtenida en pacientes negativos para HIV o con estatus desconocido (9,3 %). Al estratificar los frotis en directos y concentrados, se mantuvo la superioridad de la MF-LED. Las especificidades de la MF-LED (99,9 %) y la M-ZN (99,9 %) resultaron elevadas. La lectura de una muestra de frotis mostró una positividad significativamente mayor con un aumento de 200X (49,4 %) que con uno de 400X (33,8 %) (p<0,05). El dispositivo LED tuvo una buena aceptación entre los técnicos.
Conclusión. Debido al mejor desempeño de la MF-LED comparada con la M-ZN en pacientes con HIV y su fácil utilización, se recomienda su adopción. La utilización del aumento de 200X fue esencial para el incremento de la sensibilidad de la MF-LED.
Palabras clave: tuberculosis; fluorescencia; microscopía; diagnóstico; esputo; bacilo ácido-alcohol resistente.
http://dx.doi.org/10.7705/biomedica.v37i2.3276
As of 2011, the World Health Organization has recommended the use of LED fluorescence microscopy (FM) as an alternative of Ziehl-Neelsen (ZN) in a phased manner for a more rapid tuberculosis (TB) diagnosis (1). FM offers many advantages over ZN. It can detect approximately 5-10% more acid- fast bacilli (AFB) positive smears (2) than ZN. The higher contrast of AFB fluorescence allows the screening of slides at a much lower magnification (200X or 400x) than ZN (1,000X), and they can be examined more quickly, which offers potential solutions to the high workload in some laboratories (3). Besides, FM staining quality may be easier to control because the quality of commercial auramine dye is less variable than that of basic fuchsine (4). Furthermore, the staining technique is simpler and the auramine solution is easier to prepare (5). As no immersion objective is needed to observe the bacilli, there is no need to use the immersion oil and the xylene required with ZN, which are both expensive and may damage the objectives through mishandling or due to poor quality (6). However, fluorescent microscopes using mercury-vapor lamps (MVL) are relatively expensive, they have a short life span and require a reliable electricity supply, besides, replacement bulbs may be difficult to obtain (1). These factors have led to an interest in FM using LED. Compared to conventional MVL fluorescence microscopes, LED microscopes are less expensive and have fewer maintenance requirements. The diodes are very durable, do not require warm- up time, and do not contain toxic products. More importantly, they are reported to perform equally well without a darkroom (1).
As HIV-infected patients are considered a population in whom ZN microscopy tends to produce a low yield, interventions to increase the sensitivity of microscopy are needed. Conventional MVL-FM has shown promising results in HIV-infected individuals; two relevant studies have reported that FM was significantly more sensitive for the diagnosis of pulmonary TB than ZN microscopy in this group of patients (7,8). On the other hand, accuracy data for LED-FM in HIV-infected patients are scarce (9-12); furthermore, the results of some studies are conflicting with those obtained with conventional FM, showing a similar sensitivity (10) or lower specificity (9, 11) values for LED-FM compared with ZN smear microscopy. In addition, only few studies have evaluated the possible effect of sputum processing on the performance of LED-FM, as well as the best scheme for smear examination to achieve increased sensitivity (10,12,13). Besides, as available commercial LED systems may have different operational characteristics, to scale up the replacement of ZN by LED-FM more research is needed to evaluate the different LED systems recently introduced in the market. In low and middle income countries, local production and technology transfer is a good strategy to increase access to medical devices. The TK-LED microscope lamp (Tolket S.R.L.), produced by a technology company based in Buenos Aires, Argentina, uses the latest high power LED illumination technology, it is compatible with most leading microscope brands and can be adapted to any of them on requirement.
Argentina has a well-structured TB laboratory network which performs approximately 150,000 AFB smear microscopy examinations every year. The technical quality and agreement of ZN smear microscopy has been satisfactory over the last years, but operational and epidemiological analyses have shown that there is a need of increasing the number of sputa studied by microscopy in order to prevent diagnostic delay (14,15). The replacement of light microscopy by FM would be one of the immediate options to cope with expected increases in workload, especially in high-burden settings.
This study evaluated the performance of LED-FM for the detection of AFB from respiratory samples of patients with and without HIV infection using a newer, less expensive national LED lamp mounted on an Olympus CX31 microscope, taking culture as the reference standard. We also evaluated different LED-FM technical requirements under field conditions.
Materials and methods
Participating laboratories and technicians
In 2012, seven public laboratories were included in a multicenter project to assess the feasibility of using LED-FM in Argentina. Two of these laboratories had previous experience in conventional FM, and one of them was designated as coordinating center responsible for training, monitoring, slide rechecking and data management. The project was developed in two phases: (i) The technicians training phase, and (ii) the LED-FM evaluation study itself. The first phase was held during 2012, whereas the assessment study was accomplished during 2013 and 2014.
Laboratories were selected according to their workload, HIV prevalence among TB patients and interest in the project. Besides, to be eligible the site had to have acceptable performance in ZN blinded smear rechecking process during the last three years. A ZN acceptable performance laboratory was defined as that with less than 5% error rate and no high false positive or high false negative errors.
The technicians involved in the project attended a three-day course followed by a two-month on-site training in which each technician examined slides from the same specimen stained by ZN and FM in unblinded manner in order to acquire confidence to recognize the bacillus. During this training phase, technicians proficiency was evaluated using a monthly testing panel (composed of nine negative and nine positive smears with different positivity degrees), and a monthly random blinded rechecking. Trainees were evaluated only if they showed an acceptable performance defined as follows: 1) At the most, one low false negative (LFN) error, i.e., a scanty (1-19 AFB/1 length) positive smear misread as negative, without any high false negative results (a 1+ to 3+ positive smear misread as negative) or false positive errors (FP), i.e., a negative smear misread as positive in the last proficiency testing panel, and 2) at the most two minor errors (no more than one LFP, i.e., a negative smear misread as a scanty one, 1-19 AFB /1 length) without any major error (high false positive or high false negative) in the rechecking process.
Clinical specimens included in the evaluation study and smear microscopy
This study was conducted using specimens submitted routinely to the participating laboratories for mycobacterial culture. We included a total of 6,968 diagnostic and 2,419 follow-up respiratory specimens (comprising sputum, bronchialveolar lavage, bronchial wash and lung aspirates).
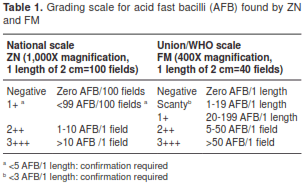
Direct or concentrated smears (16) were prepared in duplicate following the method routinely used in each participating laboratory. Hot ZN technique (0.3% carbolfuchsine and 0.1% methylene blue) (17) or 0.1% auramine O (counterstained with 0.5% potassium permanganate) for LED-FM (18) were used for staining each of these slides. ZN slides were examined with the bright field microscopy used routinely in each laboratory at 1,000X magnification, whereas FM slides were examined with Olympus CX31 microscopes with a TK-LED illumination (Tolket S.R.L., Buenos Aires, Argentina), using 200X magnification for screening and 400X magnification for confirming and quantifying the slides. The laboratory technicians were also advised to read doubtful slides using higher magnification (1,000X) for confirmation. LED-FM grading of smears was done according to WHO/IUTLD guidelines (18), whereas ZN grading was made according to national guidelines (17) (table 1). After examination, all smears were kept in a dark room at ambient temperature (23°-27°C). After smear preparation, all specimens were decontaminated, concentrated by centrifugation and the deposits were inoculated on solid and/or liquid media according to the technique routinely established in each participating laboratory and following the national tuberculosis guidelines on culture (16). The result of diagnostic samples culture was used as the reference standard. Once contaminated specimens or those in which a non-tuberculous mycobacterium was isolated were excluded, 6,637 diagnostic and 2,419 follow-up specimens were finally analyzed. HIV status data was extracted from the registers kept in the participating laboratories.

A similar proportion of slides stained by ZN and FM were read by the microscopists unaware of patients’ characteristics and comparative tests results. In one of the laboratories, in which only one technician was responsible for reading both slides, blinding was ensured by labelling FM and ZN slides with different identification numbers; besides, different registers were used to record ZN and FM results.
The external quality assurance protocol as specified by national guidelines for ZN microscopy (17) was in place in all these services. For the purpose of this multicenter evaluation, FM performance was assessed by rechecking all slides at the coordinating center. Slides were re-stained to avoid discrepancies in reading. Onsite evaluation was also carried out by technical consultants from the coordinating center during supervisory visits, and no discordances were found between laboratory technicians and supervisors results.
During all the training phases, patient care was based on the results of ZN examination and, therefore, laboratory comparisons did not affect routine patient management or involved collection of additional samples, so individual informed consent was not necessary.
Comparison of examination schemes
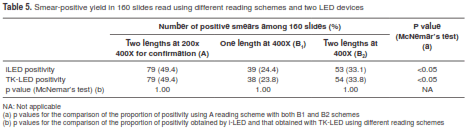
This evaluation was conducted at the coordinating center: A sample of 160 slides previously read using TK-LED was selected by picking all scanty positive slides detected during the study period at the coordinating center laboratory, as well as re-stained and re-read in blinded manner using two different examination schemes: (A) Two lengths at 200X magnification with 400X confirmation, and (B) one or two lengths at 400X. Two distinct LED devices, TK-LED and Primostari-LED (Carl Zeiss the immediately next smear-negative slide. The slides were labelled with a different identification, Jena, Germany), were used to re-read these slides in a blinded manner. The decision of enriching our sample with low positive smears (50% of scanty and 50% of negative slides) was made in an effort to target those slides most at risk of being missed by the different reading schemes, in case there would be any difference in the accuracy of both checking diagrams. We chose to evaluate the schemes by using the Zeiss device in conjunction with TK-LED microscope considering the wide acceptance of the Zeiss microscope in global evaluations.
End-user appraisal
A qualitative end-user appraisal survey was conducted among laboratory technicians after approximately three months of experience with LED-FM. The questionnaire included rating of signal-noise ratio, uniformity and intensity of fluorescent illumination, ease of focusing and scale-up suitability.
Statistical analysis
Sensitivity and specificity values were calculated for FM and ZN using culture as the reference standard for diagnosis specimens. Those specimens contaminated in culture were excluded for the analysis as diagnosis could not be made with certainty. We calculated ZN and FM positivity rates for specimens collected from follow-up patients on anti-tuberculosis treatment.
Statistical analysis was performed using the Epidat 3.1, version (PAHO) and Medcalc (MedCalc Software bvba). Results were considered significant at p<0.05. Comparison of sensitivity, specificity and positivity rates in samples from follow-up patients where both methods were applied to the same specimen were done using the McNemar’s test. We also calculated positive and negative predictive values with 95% confidence intervals (CI) for each microscopy test.
Subgroups were set by HIV infection and by concentrating or not specimens before smear preparation; a chi-square test was used to compare sensitivities in subgroups.
To compare semi-quantitative microscopy results with LED-FM and ZN, we calculated CI, and statistical significance was defined as non-overlapping 95% CI.
McNemar’s test was used to compare ZN and LED- FM positivity obtained by different examination schemes or using different LED devices applied to the same sample of smears.
Ethical review was waived as there was no potential risk to participants’ safety, privacy or confidentiality since no formal contact occurred between investigators and participants either directly (interview, questionnaires, etc.) or indirectly (medical records, personal identifiers, etc.). Data on respiratory specimens provided for routine clinical care services were completely anonymized before their inclusion in the study.
Results
LED-FM and ZN performance
Out of the 6,637 diagnostic specimens with microscopy results, culture and species confirmation, 869 (12.5%) were culture-positive for M. tuberculosis complex. ZN and LED-FM performance in the seven participating laboratories is shown in table 2. Overall, the sensitivity of LED-FM was 11.4% higher than that of ZN (87.7%; 762/869 vs. 76.3%, 663/869; McNemar’s test: p<0.001), while the levels of specificity obtained with both methods were high and quite similar (5,764/5,768; 99.9% for ZN vs. 5,763/5,768; 99.9% for LED-FM; McNemar’s test: p=1). We found a statistically significant difference in sensitivity between the two methods in all laboratories (McNemar’s test: p<0.05) except for laboratory #5 (McNemar’s test: p=0.22).The overall positive and negative predictive values for M. tuberculosis detection by LED-FM were 99.4% (95%CI 98.4-99.8) and 98.2% (95%CI 97.8-98.5), respectively, whereas the corresponding values for ZN were 99.4% (95%CI 98.4-99.8) and 96.6% (95%CI 96.0-97.0), respectively.
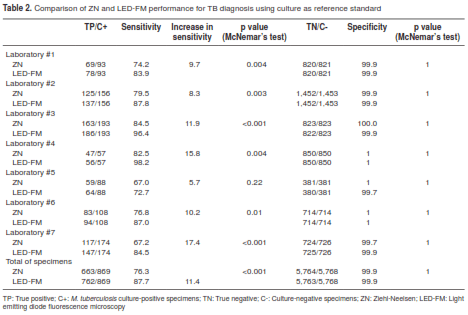
In follow-up patients, the positivity rate for LED-FM (14.2%; 344/2,419) was 3.2% higher than for ZN (11.0%; 267/2,419; p<0.001 McNemar’s test).
Diagnostic sensitivity in HIV-infected individuals
Table 3 shows that LED-FM was more sensitive than ZN, irrespective of HIV status (p<0.001 for the comparison of sensitivity values of LED- FM vs. ZN in both HIV positive or HIV negative/ unknown patients, McNemar’s test). Overall, both ZN and LED-FM had a lower sensitivity among HIV-infected patients compared with most probably HIV-negative patients (59.4% vs. 80.1% for ZN in HIV-infected patients and HIV-negative patients, respectively, chi-square test: p<0.001; 80.0% vs. 89.4% for LED-FM in HIV-positive patients and HIV-negative patients, respectively, chi-square test: p=002) (table 3.)
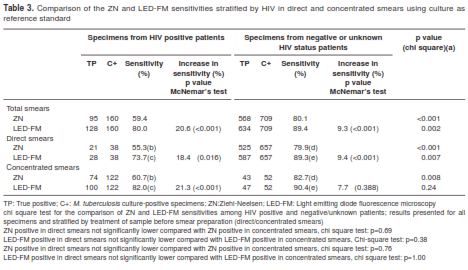
HIV positive and negative culture-positive specimens were stratified according to the method used for smear preparation. As shown in table 3, both ZN and FM sensitivities seemed slightly lower, though not significantly, in direct smears than in concentrated smears; among HIV patients, ZN direct and concentrated smear sensitivities were 55.3% vs. 60.7%, respectively (chi-square test: p= 0.69), whereas values for LED-FM using direct and concentrated smears were 73.7% and 82.0%, respectively (chi-square test: p= 0.38). Similarly, among most probably negative HIV-patients, ZN sensitivity was slightly lower using direct smears (79.9%) in comparison with concentrated smears (82.7%) (chi-square test: p= 0.76), while LED-FM sensitivities using direct or concentrated smears were very similar (89.3% vs. 90.4%, respectively, chi-square test: p=1). In the group of HIV-infected patients, differences between LED-FM and ZN sensitivities remained almost equivalent, regardless if specimens were concentrated or not before smear preparation. On the other hand, although we observed a better LED-FM performance in the group of most probably HIV-negative patients using direct or concentrated smear as compared with ZN, such difference was not statistically significant in the subgroup of concentrated smears (82.7% vs. 90.4% for ZN and LED-FM sensitivities, respectively; McNemar’s test: p=0.39), possibly due to the small number of culture-positive specimens available in this category, which resulted in wide confidence intervals around estimates of diagnostic sensitivity.
Comparison of microscopy results with LED- FM and ZN
A total of 227 specimens had discrepant results: 25 slides were positive using ZN and negative using LED-FM. Another 202 slides that were positive in LED-FM were reported negative using ZN (table 4).
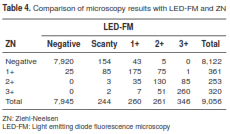
When low positive smears (scanty and +) were analyzed, 197 of the 504 (39.1%, 95% CI 34.9-43.4%) detected by LED-FM were negative by ZN, while 25 of the 361 (6.9%, 95% CI 4.7-10.2%) detected by ZN were negative by LED-FM.
Overall, there was an increase in the proportion of positivity in all categories of smear quantification with the use of LED-FM; of the 9,056 specimens included in the study, the number of positives increased from 361 slides (4.0%; CI 3.6-4.4%) with ZN to 504 smears (5.6%; CI 5.1-6.1%) with LED-FM for low positive smears, and from 573 slides (6.3%; CI 5.8%-6.8%) with ZN to 607 smears (6.7%; CI 6.2-7.2%) with LED-FM for high positive smears (++ and +++).
Comparison of examination schemes
There was no difference in smear-positive detection yield between TK-LED and Primostari-LED; (McNemar’s test: p=1) (table 5). On the contrary, the proportion of positivity with both microscopes was significantly higher when using reading scheme A (two length at 200x/ 400x for confirmation) in comparison with examining one or two lengths at 400x magnification (scheme B) (table 5).
End-user appraisal
All the fourteen technicians participating in the study gave positive feedback on TK-LED device signal- noise ratio, uniformity of fluorescent illumination and ease to focus. All believed that the use of LED- FM could improve examination speed and that the TK-LED microscope could be used without a darkroom. All technicians said that LED-FM could be scaled up, and twelve thought that laboratories with a big daily workload should be given priority in the employment of LED-FM. No technical problems were reported during the 36 months of TK-LED device usage both for the study and in routine practice in two laboratories.
Discussion
Our findings further confirm the previously reported better performance of LED-FM over the conventional ZN technique (19,20,21). A significant increased sensitivity of LED-FM over ZN was found in all laboratories except in laboratory #5. Although it is well known that microscopy performance is highly dependent upon factors such as the setting and the population attended in the service (22,23), we identified no factor that could bias the results in favor of ZN performance in laboratory #5. On the other hand, it is unlikely that such result could be explained by a poor proficiency of the technicians working in this service, as they showed an acceptable performance during the training phase and the rechecking process. Furthermore, given the small sample size in this laboratory, the power of the statistical test was about 35%, which suggests that there was not a very high chance of detecting differences between both tests, even though LED- FM showed a higher sensitivity.
Among HIV patients, two relevant studies (7,8) performed with conventional FM reported increased sensitivity over ZN. Nevertheless, although published studies have shown that LED microscopy works as well as conventional FM, both in research and in operational settings (19,24,25), the few studies evaluating LED-FM utility for diagnosing TB in HIV- infected people have shown doubtful results. Two studies (9,11) have reported a similar sensitivity of LED-FM compared to ZN, and one of them, conducted in Indonesia(11), showed that FM was less specific than ZN. Conversely, our results are in line with those performed with conventional FM, showing that among HIV-positive TB patients, the difference in sensitivity between LED-FM and ZN (20.6%) doubled that obtained in the group of TB patients with negative or unknown HIV status (9.3%). However, a recent report (26) showed that LED-FM sensitivity in the HIV-infected population continued to be lower than that obtained in most probably HIV-negative patients, possibly due to the higher occurrence of paucibacillary TB in HIV population and the difficulties in obtaining good quality sputum specimens in advanced cases (27). As various methods of concentrating sputum based on centrifugation have been shown to increase diagnostic yield when used prior to microscopy (28), the use of direct or concentrated smears could behave as a confounding factor when evaluating LED-FM performance in different populations.
In agreement with results recently reported by Getachew, et al.(12), when we stratified smears by their direct/concentrated state, the diagnostic superiority of LED-FM over ZN remained equivalent. FM appears to be a more sensitive technique than ZN due to its ability to detect low bacillary load in the sputum, as previously reported (11,21). In accordance with these studies, we found that the use of LED-FM significantly increased the proportion of low positive smears in about 40.0% compared with ZN. A stronger absorbability of mycolic acid by auramine-O than by fuchsine would partially explained the increased sensitivity of LED-FM for AFB. Furthermore, according to results by other researchers (20,29), applying both microscopy techniques to follow-up sputum, expected to have an increased proportion of scanty results compared to diagnostic sputum (30), the positivity rate with LED- FM was significantly higher than with ZN. Other factors that contribute to this higher performance of LED-FM, such as improvement in the proficiency of the laboratory technicians due to additional training in FM and new microscopes, may have played a role to a lesser extent, especially considering that one of the conditions for including a laboratory in this multicenter study was an acceptable performance in the ZN external quality assessment during the last three years.
We found that even LED-FM had a higher sensitivity than ZN, as 25 ZN-positive specimens resulted negative by LED-FM. This may be explained by two phenomena: First, as AFB are not homogeneously distributed in sputum, the portion of sputum used to prepare ZN-stained slide may have contained them, whereas the portion used to prepare the slide stained with auramine may have not, especially in those specimens with scarce bacilli. On the other hand, the uneven distribution of AFB on the slide may also explain this phenomenon; as technicians can only observe a proportion of fields on a slide, some AFB may have not been detected simply because the technicians examined those fields that were randomly AFB-depleted; this can explain why different laboratory professionals examining the same number of fields on a slide may get differing results, as it has been previously reported (31).
The larger field area subject to examination using FM compared to the oil immersion fields needed when ZN is used may also explain FM higher sensitivity. The field of view when a 20x objective is used is three to four times larger than that of the 40x objective and 25 times larger than that of the 100x objective used for ZN microscopy (32). Accordingly, we found that, in agreement with Kubica’s study (33) using conventional FM, scanning with 200x magnification enabled a higher positivity yield than examining smears at 400x only, while AFB remained clearly visible with both of the stand alone FM systems used in this study. On the contrary, other authors have reported that using two LED modules mounted on routine microscopes screening smears under 200x magnification did not increase sensitivity compared to screening under 400x magnification (34,35). These authors also reported that with these devices AFB were less clearly visible under 200x compared to 400x magnification, which may explain the differences between their results and ours when LED-FM technology but different devices were evaluated.
The impossibility of comparing TK-LED and Primostari-Led performance was a limitation of our study. To ensure a more precise comparison of positive detection by the two LEDs using different reading schemes, we analyzed a random selection of low positives and the same number of negatives detected with TK-LED. In this small fraction of TK-LED negative slides, no false negatives were detected using the i-LED device. However, given the low proportion of negatives evaluated with both microscopes, it does not seem unreasonable to assume that no TK-LED false-negatives would have been detected if all the negatives had been reread with the i-LED microscope. In these circumstances we were able to compare the detection capacity with the two reading schemes, but not the overall performance of the two LED devices.
Concerns have been voiced regarding the possibility of false positive smears with LED-FM (due to impurities in auramine, food particles and artifacts which produce some fluorescence), and it has been suggested that all scanty and doubtful cases must be confirmed by ZN (36). However, this tends to oversee the increased sensitivity and efficiency gained with the use of LED-FM, which some experts have recommended to discourage (37). The level of specificity of LED-FM in our study was high and similar to that obtained with ZN. Conversely, in their evaluation of LED-FM in HIV-infected patients, Chaidir, et al. (11), reported a significantly lower specificity compared to ZN microscopy, and they pointed out that false positive LED-FM results may have been due to lack of experience or training among technicians. Albert, et al. (9), reported a marked difference in the specificity of LED-FM among readers recently trained to evaluate LED- FM performance in HIV-infected patients, which highlights the importance of implementing careful quality assurance measures when a new technology is introduced.
Our study had these additional limitations: (i) it was performed by specimen and not by patient, and while this is consistent with most other studies in this field, we acknowledge that the lack of independence between specimens from the same patient may have led to overestimate accuracy, and (ii) HIV testing was not done systematically as part of the study; according to laboratory records, no HIV status had been registered for a significant number of patients, which underpowered the result analysis by HIV status.
Regarding feasibility aspects, we confirmed the wide acceptance of LED-FM by technicians and the possibility of using it without a dark room. We also found that new national instruments worked well during the first three years. These are all very significant factors in scaling-up LED-FM in national TB programs with limited resources.
To conclude, our findings confirmed LED-FM better performance compared to ZN in HIV-infected patients as previously recorded for conventional FM. Furthermore, user appraisal supported LED- FM rapid roll-out, especially when the burden of HIV-infected population is high. The use of 20x objective for screening was essential to achieve increased sensitivity. WHO has recently endorsed the implementation of Xpert MTB/RIF assay as the first initial TB screening in HIV patients (38), however, microscopy is still needed for treatment monitoring. In their comparison of LED-FM and Xpert MTB/ RIF assay in HIV patients, álvarez-Uria, et al. (39), reported that although the molecular assay showed an increase in positive results in comparison with LED-FM when performing both tests in the same specimen, this increase was only modest when analyzing two specimens by LED-FM. Besides, the cost of the equipment, the annual maintenance and the consumables are considerably higher for the Xpert MTB/RIF assay, and this could hinder its use in some resource-limited settings. The 2015 TB Report (40) granted that LED-FM adoption remained low; in 2014, this technology was reported to be present in only 2% of microscopy centers in the Region of the Americas. In this sense, we hope our findings are useful to increase the implementation of this technology. Particularly in Argentina, the operational and technical evidences we provided will be a valuable input in the preparation of a national strategic plan to expand the use of LED-FM.
Acknowledgments
Group of Implementation of Fluorescence Microscopy: Mónica Boutonnet , Viviana Caserío , Ana Etchart 3, Sandra Fajardo2, Mónica García5, Noemí Gómez4, María Gustincic6, Viviana Izquierdo3, Arnaldo Jara4, Graciela Kozicky2, Mario Matteo5, Carlos Pellegrini2, Silvia Pellegrino4, Sebastián Pérez-Catalán6, Carina Sacramone4, Gabriela Santiso6, Sandra Vilche1, Daniel Eletti1
1 Instituto Nacional de Enfermedades Respiratorias “Emilio Coni”, ANLIS “C.G. Malbrán”, Santa Fe, Argentina
2 Dirección de Bioquímica de la Municipalidad de Rosario, Rosario, Argentina
3 Hospital San Roque, San Salvador de Jujuy, Argentina
4 Laboratorio Central de Salud Pública, Resistencia, Argentina
5 Hospital “Dr. Francisco Javier Muñiz”, Ciudad Autónoma de Buenos Aires, Argentina
6 Hospital Interzonal General de Agudos “Evita”, Lanús, Argentina
7 Hospital Rawson, Córdoba, Argentina
Conflicts of interest
The authors have declared that no conflicts of interest exist.
Financial support
This study received financial support from the Agencia Nacional de Promoción Científica y Tecnológica through its Public Health Service grants (PAE-PID-2007-00127).
Corresponding author:
María Imaz, Instituto Nacional de Enfermedades Respiratorias “Emilio Coni”, Administración Nacional de Laboratorios e Institutos de Salud “C.G. Malbrán”, Avenida Blas Parera 8260, 3000, Santa Fe, Argentina Teléfono y fax: (54) (342) 489 2830, extensión 3 suimaz@yahoo.com
References
1. World Health Organization. Fluorescent Light-Emitting Diode (LED) microscopy for diagnosis of tuberculosis: Policy statement. Accessed: February 4, 2016. Available from: http://apps.who.int/iris/bitstream/10665/44602/1/9789241501613_eng.pdf?ua=1&ua=1
2. Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;6:570-81.
3. Bennedsen J, Larsen SO. Examination for tubercle bacilli by fluorescence microscopy. Scand J Respir Dis. 1966;47: 114-20.
4. Gordon C, van Deun A, Lumb R. Evaluating the performance of basic fuchsin for the Ziehl-Neelsen stain. Int J Tuberc Lung Dis. 2009;13:130-5.
5. van Deun A, Hossain M A, Gumusboga M, Rieder H L. Ziehl Neelsen staining: Theory and practice. Int J Tuberc Lung Dis. 2008;12:108-10.
6. Lumb R, van Deun A, Kelly P, Bastian I. Not all microscopes are equal. Int J Tuberc Lung Dis. 2006;10:227-9.
7. Kivihya-Ndugga LE, van Cleeff MR, Githui WA, Nganga LW, Kibuga DK, Odhiambo JA, et al. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. Int J Tuberc Lung Dis. 2003;7:1163-71.
8. Prasanthi K, Kumari AR. Efficacy of fluorochrome stain in the diagnosis of pulmonary tuberculosis co-infected with HIV. Indian J Med Microbiol. 2005;23:179-81.
9. Albert H, Nakiyingi L, Sempa J, Mbabazi O, Mukkada S, Nyesiga B, et al. Operational implementation of LED fluorescence microscopy in screening tuberculosis suspects in an urban HIV clinic in Uganda. PLoS One. 2013;8:e72556. http://dx.doi.org/10.1371/journal.pone.0072556.
10. Whitelaw J, Peter H, Sohn D, Viljoen G, Theron M, Badri V, et al. A comparative cost and performance of light- emitting diode microscopy in HIV–tuberculosis-coinfected patients. Eur Respir J. 2011;38:1393-7. http://dx.doi.org/10.1183/09031936.00023211
11. Chaidir L, Parwati I, Annisa J, Muhsinin S, Meilana I, Alisjahbana B, et al. Implementation of LED fluorescence microscopy for diagnosis of pulmonary and HIV-associated tuberculosis in a hospital setting in Indonesia. PLoS One. 2013;8:e61727. http://dx.doi.org/10.1371/journal.pone.0061727
12. Getachew K, Abebe T, Kebede A, Mihret A, Melkamu G. Performance of LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis in HIV positive individuals in Addis Ababa, Ethiopia. Tuberc Res Treat. http://dx.doi.org/10.1155/2015/794064
13. Bonnet M, Gagnidze L, Guerin PJ, Bonte L, Ramsay A, Githui W, et al. Evaluation of combined LED-fluorescence microscopy and bleach sedimentation for diagnosis of tuberculosis at peripheral health service level. PLoS One. http://dx.doi.org/10.1371/journal.pone.0020175
14. Imaz MS, Sequeira MD. Bacteriological diagnosis of tuberculosis in Argentina: Results of a national survey. Cad Saúde Pública. 2007;23:885-96. http://dx.doi.org/10.1590/S0102-311X2007000400016
15. Zerbini E, Chirico MC, Salvadores B, Amigot B, Estrada S, Algorry G. Delay in tuberculosis diagnosis and treatment in four provinces of Argentina. Int J Tuberc Lung Dis. 2008;12:63-8.
16. Ministerio de Salud de Argentina. Manual para el diagnóstico bacteriológico de tuberculosis. Parte II. Cultivo. Buenos Aires: Ministerio de Salud; 2007.
17. Ministerio de Salud de Argentina. Manual para el diagnóstico bacteriológico de tuberculosis. Parte I. Baciloscopia. Santa Fe: Instituto Nacional de Enfermedades Respiratorias “E. Coni”; 2012.
18. Rieder H L, van Deun A, Kam K M, Kim S J, Chonde T M, Trébucq A, et al. Priorities for tuberculosis bacteriology services in low-income countries. Second edition. Paris: International Union against Tuberculosis and Lung Disease; 2007.
19. van Deun A, Chonde M, Gumusboga M, Rienthong S. Performance and acceptability of the FluoLED Easy module for tuberculosis fluorescence microscopy. Int J Tuber Lung Dis. 2008;12:1009-14.
20. Xia H, Song YY, Zhao B, Kam K-M, O’Brien RJ, Zhang Z, et al. Multicentre evaluation of Ziehl-Neelsen and light- emitting diode fluorescence microscopy in China. Int J Tuber Lung Dis. 2013;17:107-12. http://dx.doi.org/10.5588/ijtld.12.0184
21. Reza LW, Satyanarayna S, Enarson DA, Kumar AMV, Sagili K, Kumar S, et al. LED-Fluorescence microscopy for diagnosis of pulmonary tuberculosis under programmatic conditions in India. PLoS ONE. 2013;8:e75566. http://dx.doi.org/10.1371/journal.pone.0075566
22. Perkins M D, Roscigno G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942-3.
23. Mambo-Muvunyi C, Masaisa F, Bayingana C, Musemakweri A, Mutesa L, Carbonell-Hernández T. Prevalence and diagnostic aspects of sputum smear positive tuberculosis cases at a tertiary care institution in Rwanda. Afr J Microbiol Res. 2010;4:88-91.
24. Marais BJ, Brittle W, Paincyzk K, Hesseling AC, Beyers N, Wasserman E, et al. Use of light-emitting diode fluorescence microscopy to detect acid-fast bacilli in sputum. Clin Infect Dis. 2008;47:203-7.
25. Trusov A, Bumgarner R, Valijev R, Chestnova R, Talevski S, Vragoterova C, et al. Comparison of Lumin LED fluorescent attachment, fluorescent microscopy and Ziehl-Neelsen for AFB diagnosis. Int J Tuber Lung Dis. 2008;13:836-41.
26. Chang EW, Page AL, Bonnet M. Light-emitting diode fluorescence microscopy for tuberculosis diagnosis: A meta-analysis. Eur Respir J. 2016;47:929-37. http://dx.doi. org/10.1183/13993003.00978-2015
27. Elliott AM, Halwiindi B, Hayes RJ, Luo N, Tembo G, Machiels L, et al. The impact of human immunodeficiency virus on presentation and diagnosis of tuberculosis in a cohort study in Zambia. J Trop Med Hyg. 1993;96:1-11.
28. Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;6:664-74. http://dx.doi.org/10.1016/S1473-3099(06)70602-8
29. Thapa B, Reza LW, Kumar AM, Pandey A, Satyanarayana S, Chadha S. Light Emitting Diode Fluorescence Microscopy increased the detection of smear-positives during follow-up of tuberculosis patients in India: Program implications. BMC Res Notes. 2015;8:596. http://dx.doi.org/10.1186/s13104-015-1584-z
30. Otero L, van Deun A, Agapito J, Ugaz R, Prellwitz G, Gotuzzo E, et al. Quality assessment of smear microscopy by stratified lot sampling of treatment follow-up slides. Int J Tuberc Lung Dis. 2011;15:211-6.
31. APHL/CDC/IUATLD/KNCV/RIT/WHO. External quality assessment for AFB smear microscopy. Washington, D.C.: APHL; 2002.
32. Smithwick RW. Laboratory manual for acid-fast microscopy. Atlanta, GA, USA: US Public Health Service; 1976.
33. Kubica GP. Correlation of acid-fast staining methods with culture results for mycobacteria. Bull Int Union Tuberc. 1980;55:117-24.
34. Affolabi D, Torrea G, Odoun M, Senou N, Ali Ligali M, Anagonou S, et al. Comparison of two LED fluorescence microscopy build-on modules for acid-fast smear microscopy. Int J Tuberc Lung Dis. 2010;14:160-9.
35. Bonnet M, Gagnidze L, Githui W, Guerin PJ, Bonte L, Varaine F, et al. Performance of LED-based fluorescence microscopy to diagnose tuberculosis in a peripheral health centre in Nairobi. PLoS ONE. 2011;6:e17214. http://dx.doi. org/10.1371/journal.pone.0017214.
36. Das D, Selvakumar N. Can LED fluorescence microscopy replace Ziehl-Neelsen microscopy in tuberculosis detection? Int J Tuberc Lung Dis. 2012;16:1558. http://dx.doi.org/10.5588/ijtld.12.0407
37. van Deun A, Cattamanchi A, Davis JL, Ridderhof J. In reply. Can LED fluorescence microscopy replace Ziehl- Neelsen microscopy in tuberculosis detection? Int J Tuberc Lung Dis. 2012;16:1558-9. http://dx.doi.org/10.5588/ijtld.12.0407-2.
38. World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test. WHO/HTM/TB/2011.2. Geneva: WHO; 2011. Accessed: February 4, 2016. Available from: http://www.who.int/tb/features_archive/xpert_rapid_tb_test/en/
39. álvarez-Uria G, Azcona JM, Midde M, Naik PK, Reddy S, Reddy R. Rapid diagnosis of pulmonary and extrapulmonary tuberculosis in HIV-infected patients. Comparison of LED fluorescent microscopy and the Gene Xpert MTB/RIF assay in a District hospital in India. Tuberc Res Treat. 2012;932862. http://dx.doi.org/10.1155/2012/932862
40. World Health Organization. Twentieth global report on tuberculosis. WHO/HTM/TB/2015.22. Geneva: World Health Organization; 2015. Accessed: February 15, 2016. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.