Biomédica 2017;37(Supl.2):50-8
doi: https://doi.org/10.7705/biomedica.v34i2.3379
ORIGINAL ARTICLE
1 Centro de Investigaciones en Enfermedades Tropicales, CINTROP, Departamento de Ciencias Básicas, Facultad de Medicina, Universidad Industrial de Santander, Bucaramanga, Colombia
2 Laboratorio de Química Orgánica y Biomolecular, LQOBio, Departamento de Ciencias, Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia
Author’s contributions:
Vladimir V. Kouznetsov designed and synthesized the girgensohnine analogues.
Juliana Cuadros-Martínez carried out laboratory procedures, performed analyses and interpreted the data.
Jonny E. Duque performed analyses and interpreted the data.
Aurora L. Carreño participated in all stages of the research.
All authors participated in the writing of the paper.
Received: 22/06/16; accepted: 02/05/17
Introduction: The alkaloid girgensohnine has been used as a natural model in the synthesis of new alkaloid-like alpha-aminonitriles with insecticidal effect against disease vectors.
Objective: To evaluate the biocide activity of girgensohnine analogues and essential oils of Cymbopogon flexuosus, Citrus sinensis and Eucalyptus citriodora in stage I and stage V Rhodnius prolixus nymphs.
Materials and methods: We used a topical application model in tergites and sternites, as well as exposure to treated surfaces with different exploratory doses of each of the molecules and essential oils to determine the lethal doses (LD50 and LD95).
Results: Analogue 3 showed the highest insecticidal activity with 83.3±16.7% of mortality when applied on tergites, 38.9±4.8% on sternites and 16.7±0% on treated surfaces in stage I nymphs at 72 hours (h) and 500 mg.L -1. In stage V nymphs, the compounds induced mortality only in sternums (11.1±9.6% for analogue 6 and 5.5±4.7% for analogues 3 and 7 at 72 h and 1500 mg.L -1). The lethal doses for molecule 3 on tergites in stage I nymphs were LD50 225.60 mg.L -1 and LD95 955.90 mg.L -1. The insecticidal effect of essential oils was observed only in stage I nymphs, with 11.1±4.8% for C. flexuosus when applied in sternites, while using exposure to surfaces treated it was 5.6±4.8% for C. sinensis applied on tergites and 8.3±0% on sternites at 72 h and 1000 mg.L -1.
Conclusion: Synthetic girgensohnine analogues, and C. flexuosus and C. sinensis essential oils showed insecticidal activity in R. prolixus. Analogue 3 showed the greatest insecticidal activity among all molecules and oils evaluated under our laboratory conditions.
Key words: Triatominae; Chagas disease; insecticides; oils, volatile.
doi: https://doi.org/10.7705/biomedica.v34i2.3379
Acción insecticida de análogos sintéticos de girgensohnina y de aceites esenciales sobreRhodnius prolixus (Hemiptera: Reduviidae)
Introducción. El alcaloide natural girgensohnina se ha usado como modelo en la síntesis de nuevos análogos de alcaloidales alfa-aminonitrílicos con efecto insecticida en vectores de enfermedades.
Objetivo. Evaluar la actividad biocida de análogos de girgensohnina y de aceites esenciales de las plantas Cymbopogon flexuosus, Citrus sinensis y Eucalyptus citriodora en ninfas de estadios I y V de Rhodnius prolixus.
Materiales y métodos. Se empleó la aplicación tópica en terguitos, esternitos y superficies tratadas con diferentes dosis exploratorias de cada una de las moléculas y aceites esenciales para determinar las dosis letales (LD50 y LD95).
Resultados. El análogo 3 tuvo la mayor actividad insecticida, con una mortalidad de 83,3±16,7% en los terguitos, de 38,9±4,8 % en los esternitos y de 16,7±0 % a las 72 horas en ninfas de estadio I expuestas a superficies tratadas y 500 mg.L -1. En las ninfas de estadio V solo se presentó mortalidad en los esternitos (11,1±9,6 % con el análogo 6 y 5,5±4,7 % con los análogos 3 y 7 a las 72 h y 1.500 mg.L -1). Las dosis letales para la molécula 3 en los terguitos de ninfas de estadio I fueron las siguientes: DL50, 225,60 mg.L -1 y DL95, 955,90 mg.L -1. En cuanto a los aceites esenciales, el efecto insecticida solo se presentó con C. flexuosus (11,1±4,8%) en los esternitos de ninfas de estadio I expuestas a superficies tratadas; con C. sinensis (5,6±4,8%) en los terguitos y en los esternitos (8,3±0%) a las 72 horas y 1.000 mg.L -1.
Conclusión. Los análogos sintéticos del alcaloide girgensohnina y los aceites esenciales de C. flexuosus y C. sinensis exhibieron actividad insecticida en R. prolixus. El análogo 3 exhibió la mayor actividad insecticida de todas las moléculas evaluadas bajo las condiciones de laboratorio.
Palabras clave: Triatominae; enfermedad de Chagas; insecticidas; aceites volátiles.
doi: https://doi.org/10.7705/biomedica.v34i2.3379
According to the World Health Organization (WHO), Chagas disease or American trypanosomiasis, caused by the parasite Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae), affects the population in 21 Latin American countries. In places where this disease is endemic, the main mode of transmission is through contact with the feces of infected bloodsucking reduviid bugs (Hemiptera: Reduviidae: Triatominae) (1-3) when the affected person rubs and spreads them to the eyes, the mouth or an open skin lesion (4). At present, there is no vaccine against the etiological agent of the disease and existing drugs are only partially effective with severe side effects (4,5).
There are approximately 151 described species of triatomines grouped into 15 genera and five tribes (6). The most important domiciled species involved in the transmission of T. cruzi are: Triatoma infestans (Klug, 1834) (in the Southern Cone countries), Rhodnius prolixus Stål, 1859 (in Central America and northern South America), and Triatoma dimidiate Latreille, 1811, Phyllosoma complex, (7,8) (throughout the southern, central and eastern areas of the Gulf of México extending to Ecuador and northern Perú).
The estimated prevalence of trypanosomiasis in Colombia ranges from 700,000 to 1,200,000 inhabitants infected and 8 million at risk of acquiring the infection (9). The main vectors adapted to human habitations are, in order of importance, R. prolixus in the Andes, Caribbean, Orinoco and Amazon regions; T. dimidiate in the Andes, Caribbean and Orinoco regions; T. venosa (Stål, 1872) in the Andes and Orinoco regions; T. maculata (Erichson,1878) in the Andes, Caribbean and Orinoco regions (10), and Pastrongylus geniculatus (Latreille, 1811) in the Andes, Caribbean, Orinoco and Amazon regions (11-13). The department of Santander is one of the main endemic zones in Colombia.
The most effective method to prevent Chagas disease in Latin America is vector control (10) through conventional use of insecticides such as organochlorines, organophosphates, carbamates and pyrethroids (2). It is urgent to find natural active ingredients or new alkaloid-like molecules to replace these insecticides (14,15), as their constant use has generated resistance mechanisms in Triatomine species of epidemiological importance (2). Among these new molecules, synthetic analogues of the girgensohnine alkaloid structure were designed and synthesized to inhibit the activity of acetylcholinesterase (AChE).
According to Carreño, et al. (16), these analogues, derivatives of alpha-aminonitriles, showed insecticidal activity on larvae of Aedes aegypti, the vector that transmits dengue disease, and the authors suggest this is a strong indication of its possible effect on other insects of medical interest, such as triatomines. Essential oils are another potential tool for insect control, due to their selectivity and minimal environmental effects and the fact that, in some cases, their action has been effective against insects that are resistant to commercial insecticides (14).
In this context, our objective was to evaluate the insecticidal activity of synthetic analogues of the girgensohnine alkaloid and of essential oils from C. flexuosus, C. sinensis and E. citriodora against stage I and V R. prolixus nymphs.
Materials and methods
Biological material
We used the reference strain of R. prolixus CINTROP -UIS. Adults were fed every eight days with blood of hens (Gallus gallus). For the bioassays we used stage I nymphs of 24 to 36 h of age and 0.2-0.3 mg of weight subjected to fasting since hatching, and stage V nymphs with 20-30 mg of weight and eight days of fasting.
Synthetic analogues and essential oils
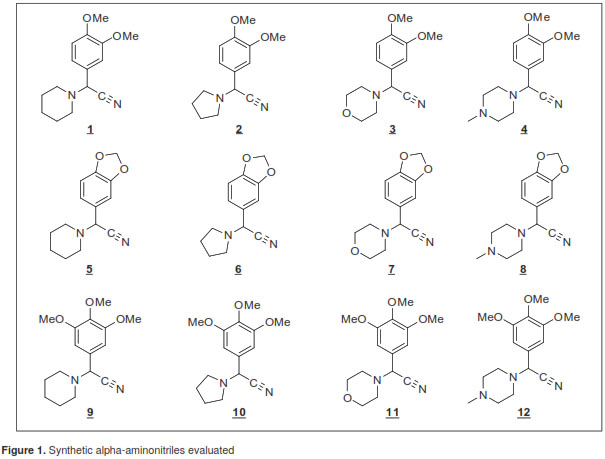
We used the girgensohnine alkaloid as a natural model in the design and synthesis of 12 new synthetic analogues (figure 1). A Strecker reaction modified from commercial and inexpensive substitute benzaldehydes (3,4-dimethoxybenzaldehyde, piperonal and 3,4,5-trimethoxybenzaldehyde), four cyclic secondary amines (piperidine, pyrrolidine, morpholine and N-methylpiperazine), and KCN and acetone cyanohydrin were employed.

In general, all molecules were prepared with moderate to excellent yields presenting welldefined melting points, and their structural elucidation was made using diverse spectroscopic techniques (FT-IR, GC-EM, 1 H NMR and 13 C NMR) (16). As positive controls of the analogues we used technical grade insecticides: Deltamethrin (99.5%-ChemService), pyrethroid used to control Rhodnius prolixus vectors in rural and peri-urban areas in Santander, and fenitrothion (98.0%-Dr. Ehrenstorfer), organophosphate used for vector control in Latin America. The essential oils of C. flexuosus (Poaceae) with geranial (37.5%), neral (28.2%) and geranyl acetate (10.0%) components; of C. sinensis (Rutaceae) with limonene (71.3%), linalol (5.4%), and beta-myrcene (5.0%), and of E. citriodora (Myrtaceae) with citronellal (49.3%), citronellol (13.0%), and isopulegol (12.9%) were donated by CENIVAM-UIS (17). All plants were collected in Santander. Following the protocol by Stashenko, et al. (18), we extracted and analyzed essential oils using microwave assisted hydrodistillation.
Exploratory doses
The exploratory doses used to evaluate each of the molecules were the following: for stage I nymphs, 25, 125, 500 mg.L -1 , and for stage V nymphs, 800, 1,000, 1,500 mg.L -1); essential oils doses for stage I nymphs were 30, 300, 1,000 mg.L -1, and for stage V nymphs, 2,000, 2,800, 3,500 mg.L -1 , which were used for the negative controls with acetone as solvent in all dilutions. The doses for the positive controls were deltamethrin (0.3, 0.7 and 500 mg.L -1 ) and fenitrothion (0.09, 0.1 and 500 mg.L -1 ) for stage I nymphs, and deltamethrin (1.0, 3.0 and 800 mg.L -1) and fenitrothion (0.45, 0.5 and 800 mg.L1 ) for stage V nymphs.
Topical application
We used a modified protocol (19) where nymphs were treated topically with the 12 synthetic girgensohnine analogues, the three essential oils and the two commercial insecticides diluted in acetone and applied with a Hamilton 5 and 25 µl microsyringe fitted with a repetitive discharger. We applied the treatments on tergites and sternites of each stage I and stage V nymphs by applying 0.1 and 0.5 µl of the experimental dose, respectively. We designed the experiments for the bioassay with arthropods (20). We repeated all doses four times with 12 insects per replicate, while the complete bioassay was replicated on three different days. After treatment, we placed the nymphs in 1/2 oz. plastic cups with folded paper inside and covered with perforated lids that would allow the nymphs to breathe. Mortality was recorded 2, 12, 24, 48 and 72 h following treatment.
Exposure to treated surfaces
The experiments followed the protocol (19) with modifications. We used the experimental doses in 6 cm Petri dishes provided with filter disks (Munktell) and we impregnated their surfaces evenly with a micropipette following a spiral pattern from the center toward the outside. A volume of 437 µl of the solution was used, calculated according to the protocol. After drying the impregnated surfaces, 12 nymphs were exposed per dose, i. e., 48 nymphs per bioassay, covering the Petri dishes with perforated cling wrap. Mortality was recorded 0.5, 1, 2, 12, 24, 48 and 72 h following treatment.
Lethal dose bioassays
We chose the molecule with the highest percentage of insecticidal activity from among all the molecules evaluated (synthetic analogues and essential oils) in stage I and stage V R. prolixus nymphs. Based on these results, we conducted bioassays with multiple asymmetrical doses whose dose-response mortality results were used to determine the LD50 and LD95 values.
Statistical analysis
We evaluated dose-response mortality data from each bioassay using the Kolmogorov-Smirnov test; the normality value indicated a non-normal distribution of the data. We analyzed the results using Kruskall-Wallis (KW) and Mann-Whitney (MW) nonparametric tests, with p values <0.05 considered as significant; we used multiple-comparisons tests when data were significantly different. For the molecule with the highest mortality rate, probit analyses were conducted to determine the statistical parameters, lethal doses (LD50 and LD95 ) and their respective confidence intervals. The statistical programs used were Statistics, v11, and Probit (21,22).
Ethics statement
The study was approved by the Ethics Committee of CEINCI-UIS, minute N° 12 of May 23, 2014.
Results
The synthesized analogues and essential oils induced differentiated mortality in nymph stages at 72 h in all experiments.
Evaluation of girgensohnine analogues in stage I R. prolixus nymphs
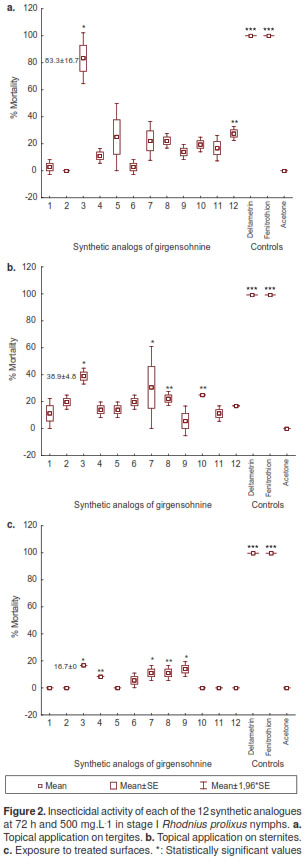
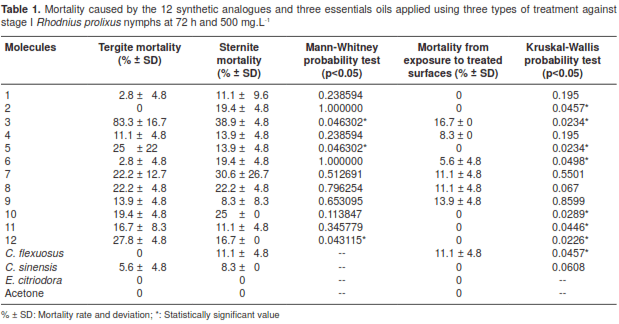
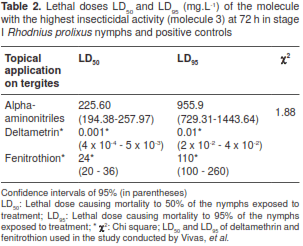
We observed insecticidal activity in 11 analogues when we used the topical application on tergites, in 12 on sternums and in six on treated surfaces, with molecule 3 showing the highest mortality rate (figure 2). Among the analogues evaluated, the mortality in tergites was significantly different (KruskalWallis: H (14 N=45)=36.51205 p=0.0009) (figure 2a). The topical application on sternites resulted in significantly low mortality (Kruskal-Wallis: H (14 N=45)=33.98065 p=0.0021) compared to tergites (table 1). The same effect was observed on treated surfaces (Kruskal-Wallis: H (14 N=45)=41.89138 p=0.0001) (figure 2c). As expected, positive (deltamethrin and fenitriothion ) and negative controls (acetone) had 100% and 0% mortality rates, respectively (figure 2). When comparing the mortality rates in the two types of treatment, tergites and sternites, there were significant differences (Mann-Whitney, MW, p<0.05) in synthetic analogues 3, 5 and 12. When comparing the three types of treatment, MW p<0.05 differences were observed in six synthetic molecules (2, 3, 5, 6, 11 and 12), and molecule 3 showed the highest mortality rate in each of the treatments (table 1), with LD50 225.60 mg.L -1 and LD95 955.90 mg.L -1 in topical application on tergites at 72 h and 500 mg.L -1 (table 2).
Evaluation of girgensohnine analogues in stage V R. prolixus nymphs
Mortality in stage V nymphs at 72 h and 1,500 mg.L -1 was low and only evidenced in sternites with 11.1±9.6% for analogue 6 and 5.5±4.7% for analogues 3 and 7, registering significantly different action (Kruskal-Wallis: H (14 N=45)=36.64743 p=0.0008) (figure 3). There was no mortality with the topical application on tergites and surfaces treated with the 12 girgensohnine synthetic analogues.
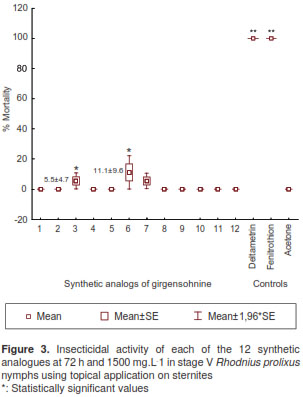
Evaluation of essential oils in stage I and V R. prolixus nymphs
We observed insecticidal activity in stage I nymphs with C. flexuosus (11.1±4.8%) on sternites, and exposed to treated surfaces at 72 h and 1,000 mg.L -1.
However, with C. sinensis at the same dose, low mortality was evidenced in tergites (5.6±4.8%) and sternites (8.3±0%). For stage V nymphs the mortality was low (8.3±0%) and it was observed only with C. sinensis in tergites. On the other hand, we registered no mortality with the topical application of C. sinensis and E. citriodora on sternites nor with the exposure to treated surfaces (table 1).
Discussion
Girgensohnine analogues
Synthetic analogues are hydrophobic molecules that, when applied topically, can easily cross the exoskeleton of stage I nymphs, a property that may be responsible for the insecticidal activity observed both in tergites and sternites. Hydrophobic sub stances penetrate the insect cuticle more easily than hydrophilic substances because they are mostly made of lipids and waxes (23-25), which are more related to the substances evaluated in our study.
However, other intrinsic and extrinsic factors (size, age, nutritional state, humidity and temperature) (26-28) can change the evaluation of the molecules. Another fact to consider is cuticle sclerotization, as nymphs of 24 to 36 hours of age have semisclerotized cuticle and, thus, higher susceptibility (28). As other authors have mentioned,differences in cuticle thickness (29) in stage I and V nymphs can affect the susceptibility to external stimuli; for instance, Reyes, et al. (30), observed that the pyrethroid insecticides (beta-cypermethrin and fenitrothion) were more effective in stage I T. dimidiata and T. maculata nymphs than in stage V ones. Cáceres, et al. (31), found that stage I R. pallescens nymphs were sensitive to deltamethrin and lambda-cyhalothrin in different geographical zones. In another study, Germano found similar results (23) in toxicity bioassays with deltamethrin in different stages of development in T. infestans.
Insecticides penetrate insects until they reach their target in three ways: through the cuticle, by ingestion and by inhalation (26,32). Penetration occurs through the cuticle via articular and inter-segmental membranes, and through spiracles. We found the highest insecticidal activity for analogue 3 when it was evaluated on tergites of stage I nymphs, suggesting that penetration occurs through the thinner cuticle of this stage in dorsal, ventral and lateral positions (28,32). On stage V nymphs molecules penetration was observed only in sternites, with lower insecticide activity given cuticle characteristics in this nymphal stage.
Synthetic analogues are non-volatile compounds that, when dissolved in a volatile solvent such as acetone, allow the formation of crystals, leaving residues in porous substrates such as filter paper. In some field studies (33-35), authors have explained the lack of persistence of substances on some porous surfaces by the fact that these surfaces retain particles, thus reducing their availability and insect-substance interaction. This may have occurred in the experiment with the Petri dishes in our study, as the insecticidal activity of the synthetic analogues on the exposed surfaces resulted in lower mortality rates. In this sense, Stampini, et al. (36), have suggested increasing the dose of the active ingredient when used on porous surfaces.
Another factor that influenced the bioactivity of the synthetic analogues was the behavior observed in stage I nymphs: they moved toward the end of the paper, where they remained throughout the bioassay, which could have decreased their interaction with the molecules.
Although the insecticidal activity of synthetic alkaloids of girgensohnine on triatomines has not yet been reported in literature, Carreño, et al. (16), reported the insecticidal activity of these analogues against Aedes aegypti, one of dengue vectors, with analogue 1 showing the highest insecticidal activity on larvae at stages III and IV. In our study, the highest insecticidal activity and mortality rates were observed for analogue 3. The LD50 and LD95 of the best molecule were high in comparison with those observed by Vivas, et al. (37), with deltamethrin and fenitrothion in susceptible strains of R. prolixus from Venezuela (table 2).
Essential oils
Low insecticidal activity of essential oils on tergites and sternites was probably due to the volatility of some of their components, which, therefore, were washed away easily by acetone in its process of evaporation following topical application, thus diminishing their bioactivity. This was also the case in the assay with treated surfaces, in which the concentration of the active compounds in the Petri dish decreased.
Some authors have compared the insecticidal activity of the topical application with the exposure to surfaces impregnated with different essential oils, and they have reported low activity with the two methods. Fournet, et al. (38), found Minthostachys andina and Hedomeam andonianum (Lamiaceae) essential oils showed low or null insecticidal activity at 72 h in stage I and V R. neglectus and T. infestans nymphs after topical application and exposure to treated surfaces.
According to Sfara, et al. (14), there was low insecticidal activity in exposure to surfaces treated with five essential oils and seven monoterpenes in stage I R. prolixus nymphs as compared with the organophosphate insecticide used as positive control. Although the essential oils evaluated in stage I nymphs showed low insecticidal activity by contact (topical application on sternites and tergites) and exposure to surfaces (fumigant effect), when ingested mortality was much higher, as has been observed in other insects. According to Hincapié, et al. (39), the extracts of Annona miricata (Anonaceae) seeds on Sitophilus zeamais (Coleoptera: Curculionidae) are more effective when ingested than by contact. Ringuelet, et al. (40), observed that Lippia alba (Verbenaceae) essential oil showed greater insecticidal activity on Tribolium castaneum (Coleoptera: Tenebrionidae) when applied on the grain, than when using the impregnation method on filter paper.
However, some authors have reported sublethal effects of essential oils on triatomines. For instance, Lutz, et al. (41), found repellent activity of some monoterpenes, methyl acetate and geraniol components of essential oils in stage V R. prolixus nymphs. The same results were reported by Zamora, et al. (42), as well as an anti-feeding effect caused by the main components of Cymbopogon winterianus (Poaceae) essential oil on three endemic species of triatomines in Arizona.
In summary, analogues of the girgensohnine alkaloid synthesized using a modified Strecker reaction showed insecticidal activity in R. prolixus nymphs. The analogue that showed the greatest insecticidal activity under laboratory conditions was molecule 3, with LD50 225.60 mg.L and LD95 955.90 mg.L in stage I nymph tergites. We observed low or no insecticidal activity in stage V nymphs with the molecules evaluated. Cymbopogon flexuosus and C. sinensis essential oils insecticidal effect was observed only in stage I nymphs, but with low activity.
We thank Prof. Dr. Elena E. Stashenko for providing the essential oils, and for their chromatographic analysis.
None declared.
This work received financial support from the Patrimonio Autónomo, Fondo Nacional de Financiamiento para la Ciencia, Francisco José de Caldas, Colciencias (contract 624/2014) and the Vicerrectoría de Investigación of Universidad Industrial de Santander-UIS (project 1389).
Corresponding author:
Jonny E. Duque, Centro de Investigaciones en EnfermedadesTropicales, CINTROP, Departamento de Ciencias Básicas, Facultad de Medicina, Universidad Industrial de Santander, Parque Tecnológico y de Investigaciones Guatiguara, km 2 Vía El Refugio, Piedecuesta, Santander, Colombia
Telephone: (577) 634 4000, extension 3526
jonedulu@uis.edu.co
1. Justi AS, Russo CA, Mallet JR, Obara MT, Galvão C. Molecular phylogeny of Triatomini (Hemiptera:Reduviida e:Triatominae). Parasit Vectors. 2014;7:149. https://doi.org/10.1186/1756-3305-7-149
2. Mougabure-Cueto G, Picollo MI. Insecticide resistance in vector Chagas disease: Evolution, mechanisms and management. Acta Trop. 2015;149:70-85. https://doi.org/10.1016/j.actatropica
3. Roca-Saumell C, Soriano-Arandes A, Solona-Díaz L, Gascón-Brustenga J, Grupo Concenso Chagas APS. Documento de consenso sobre el abordaje de la enfermedad de Chagas en atención primaria de salud de áreas no endémicas. Aten Primaria. 2015;47:308-17. https://doi.org/10.1016/j.aprim.2015.01.002
4. World Health Organization. Chagas disease (American Trypanosomiasis). Accessed: March 18, 2016. Available from: http://www.who.int/mediacentre/factsheets/fs340/en/#
5. Carabarin-Lima A, González-Vázquez MC, RodríguezMorales O, Baylón-Pacheco L, Rosales-Encina JL, ReyesLópez PA, et al. Chagas disease (American trypanosomiasis) in México: An update. Acta Trop. 2013;127:126-35. https://doi.org/10.1016/j.actatropica.2013.04.007
6. Justi SA, Galvão C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2017;33:42-52. https://doi.org/10.1016/j.pt.2016.11.002
7. Dujardin JP, Schofield CJ, Panzera F. Los vectores de la enfermedad de Chagas. Brussel: Koninklijke Academie Voor Overzeese Wetenschappen; 2002. p. 189.
8. Salazar-Schettino M, Rojas-Wastavino G, Bucio-Torres M, Martínez-Ibarra J, Monroy-Escobar M, Vences-Blanco M, et al. Revisión de 13 especies de la familia Triatominae (Hemiptera:Reduviidae) vectores de la enfermedad de Chagas en México. Journal of the Selva Andina Research Society. 2010;1:57-80.
9. Palmezano JM, Plazas LK, Rivera KE, Rueda VP. Enfermedad de Chagas: realidad de una patología frecuente en Santander, Colombia. Rev Médica UIS. 2015;28:81-90.
10. Instituto Nacional de Salud. Protocolo de Vigilancia en Salud Pública: Chagas. Accessed: August 29, 2015. Available from: http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/sivigila/Protocolos%20SIVIGILA/PRO%20Chagas.pdf.
11. Guhl F. Enfermedad de Chagas: realidad y perspectivas. Rev Biomed. 2009;20:228-34.
12. Maestre-Serrano R, Eyes-Escalante M. Actualización de la presencia y distribución de triatominos en el departamento del Atlántico-Colombia: 2003-2010. Bol Mal Salud Amb. 2012;52:125-8.
13. Parra-Henao GJ, Martínez MF, Angulo-Silva VM. Vigilancia de Triatominae (Hemiptera: Reduviidae) en Colombia. Bogotá: Colciencias-Red Chagas; 2013. p. 127.
14. Sfara V, Zerba EN, Alzogaray RA. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J Med Entomol. 2009;46:511-5. http://www.bioone.org/doi/full/10.1603/033.046.0315
15. Moretti AN, Zerba EN, Alzogaray RA. Behavioral and toxicological responses of Rhodnius prolixus and Triatoma infestans (Hemiptera: Reduviidae) to 10 monoterpene alcohols. J Med Entomol. 2013;50:1046-54. https://doi.org/10.1603/ME12248
16. Carreño-Otero AL, Vargas-Méndez LY, Duque-Luna JE, Kouznetsov VV. Design, synthesis, acetylcholinesterase inhibition and larvicidal activity of girgensohnine analogues on Aedes aegypti, vector of dengue fever. Eur J Med Chem. 2014;78:392-400. https://doi.org/10.1016/j.ejmech.2014.03.067
17. Vera SS, Zambrano DF, Méndez-Sánchez SC, RodríguezSanabria F, Stashenko EE, Duque-Luna JE. Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2014;113:2647-54. https://doi.org/10.1007/s00436-014-3917-6
18. Stashenko EE, Jaramillo BE, Martínez JR. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J Chromatogr A. 2004;1025:93-103. https://doi.org/10.1016/j.chroma.2003.10.058
19. Centro de Investigaciones de Plagas e Insecticidas. Monitoreo de la resistencia a insecticidas en Triatominos en América Latina. Anexo II: Protocolo de evalución efecto insecticida de Rhodnius prolixus. Accessed: March 17, 2017. Available from: https://www.mundosano.org/download/bibliografia/Monografia%201.pdf.
20. Robertson JL, Savin NE, Russell RM, Preisler HK. Bioassays with arthropods. Second edition. Boca Ratón: CRC Press; 2007. p. 224.
21. Finney DJ. Probit Analysis. 3rd edition. New York: Cambridge University Press; 1971. p. 333.
22. Raymond M. Présentation d’un programme d’analyse logprobit pour micro-ordinateur. Cah ORSTOM Ser Entomol Med Parasitol. 1985;23:117-21.
23. Germano MD. Herencia y efectos demográficos de la resistencia a deltametrina en Triatoma infestans.Tesis. Buenos
Aires: Universidad de Buenos Aires; 2012. Accessed: August 24, 2015. Available from: http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_5316_Germano.pdf.
24. Hadaway AB. Some factors affecting the distribution of rate of action of insecticides. Bull World Heal Organ. 1971;44:221-4.
25. Juárez MP, Fernández GC. Cuticular hydrocarbons of triatomines. Comp Biochem Physiol Part A, Mol Integr Physiol. 2007;147:711-30. https://doi.org/10.1016/j.cbpa.2006. 08.031
26. Zerba EN, de Licastro SA, Wood EJ, Picollo-de Villar MI. Modo de accion de insecticida en T. infestans. En: Carcavallo T, Rabinovich JE, Tonn RJ, editores. Factores biológicos y ecológicos en la enfermedad de Chagas. Tomo II. Párasitos-Reservorios-Control-Situación regional. Buenos Aires: OPS, OMS, Ministerio de Salud y Acción Social de la República de Argentina; 1985. p. 310-8.
27. Moretti AN. Efectos letales y subletales de monoterpenos sobre vectores de Chagas y su posible uso como herramientas de control. Tesis. Buenos Aires: Universidad de Buenos Aires; 2015. Accessed: March 20, 2017.
Available from: http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_5773_Moretti.pdf.
28. Alzogaray RA. Caracterización de la tocixidad de insecticidas piretroides en Triatoma infestans (Klug). Tesis. Buenos Aires: Universidad de Buenos Aires; 1996. Accessed: September 12, 2015. Available from: http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_2815_Alzogaray.pdf.
29. Busvine JR. A critical review of the techniques for testing insecticides. London; Imprint unknown; 1971. p. 345.
30. Reyes M, Angulo VM, Sandoval CM. Efecto tóxico de ß- cipermetrina, deltametrina y fenitrotión en cepas de Triatoma dimidiata (Latreille, 1811) y Triatoma maculata (Erichson, 1848) (Hemiptera, Reduviidae). Biomédica. 2007;27(Supl.1):75-82. https://doi.org/10.7705/biomedica.v27i1.250
31. Cáceres L, Rovira JR, Calzada J, Saldaña A. Evaluación de la actividad tóxica de los insecticidas piretroides deltametrina y lambdacihalotrina en dos poblaciones de campo de Rhodnius pallescens (Hemiptera: Reduviidae) de Panamá. Biomédica. 2011;31:8-14. https://doi.org/10.7705/biomedica.v31i1.330
32. Sugiura M, Horibe Y, Kawada H, Takagi M. Insect spiracle as the main penetration route of pyrethroids. Pestic Biochem Physiol. 2008;91:135-40.
33. Sandoval CM. Monitoreo de la resistencia a insecticidas en triatominos en América Latina. Actividad insecticida del malatión y la deltametrina en una cepa colombiana de Rhodnius prolixus (Hemimptera: Reduviidae). Accessed: August 10, 2015. Available from: https://www.mundosano. org/download/bibliografia/Monografia%201.pdf.
34. Rojas-de Arias A, Lehane MJ, Schofield CJ, Fournet A. Comparative evaluation of pyrethroid insecticide formulations against Triatoma infestans (Klug): Residual efficacy on four substrates. Mem Inst Oswaldo Cruz. 2003;98:975-80. https://doi.org/10.1590/S0074-02762003000700020
35. Palomino M, Villaseca P, Cárdenas F, Ancca J, Pinto M. Eficacia y residualidad de dos insecticidas piretroides contra Triatoma infestans en tres tipos de viviendas. Evaluación de campo en Arequipa, Perú. Rev Peru Med Exp Salud Pública. 2008;25:9-16.
36. Stampini M, Girgenti P, Locatelli DP. Activity of deltamethrin on different surfaces against Plodia interpunctella larvae (Lepidoptera: Pyralidae). Accessed: August 10, 2015. Available from: http://www.icup.org.uk/reports/icup884.pdf.
37. Vivas AS, Fernández DM. Toxicidad de cinco insecticidas en una cepa de laboratorio de Rhodnius prolixus Stål, 1859 (Hemiptera: Reduviidae) de Venezuela. Entomotropica. 2001;16:187-90.
38. Fournet A, Rojas-de Arias A, Charles B, Bruneton J. Chemical constituents of essential oils of muña, Bolivian plants traditionally used as pesticides, and their insecticidal properties against Chagas’ disease vectors. J Ethnopharmacol. 1996;52:145-9.
39. Hincapié-Llanos CA, Lopera-Arango D, CeballosGiraldo M. Actividad insecticida de extractos de semilla Annona miricata (Anonaceae) sobre Sitophilus zeamais (Coleoptera:Curculionidae). Rev Colomb Entomol. 2008;34:76-82.
40. Ringuelet JA, Ocampo R, Henning C, Urrutia MI. Actividad insecticida del aceite esencial de Lippia alba (Mill.) N. E. Brown sobre Tribolium castaneum Herbst. en granos de trigo (Triticum aestivum L.). Rev Bras Agroecol. 2014;9214-22.
41. Lutz A, Sfara V, Alzogaray RA. Repellence produced by monoterpenes on Rhodnius prolixus (Hemiptera:Reduviidae)decreases after continuous exposure to these compounds. J Insect Sci. 2014;14:254. https://doi.org/10.1093/jisesa/ieu116
42. Zamora D, Klotz EA, Meister EA, Schmidt JO. Repellency of the components of the essential oil, Citronella, to Triatoma rubida, Triatoma protracta, and Triatoma recurva(Hemiptera: Reduviidae: Triatominae). J Med Entomol. 2015;52:719-21. https://doi.org/10.1093/jme/tjv03