Efectividad in vitro de tres fármacos aprobados y su interacción sinérgica contra Leishmania infantum
Resumen
Introducción. La leishmaniasis sigue siendo una de las enfermedades tropicales desatendidas. La reutilización de medicamentos existentes ha demostrado ser exitosa para tratar enfermedades tropicales desatendidas y la terapia combinada es una alternativa estratégica para el tratamiento de enfermedades infecciosas.
Auranofin, lopinavir/ritonavir y sorafenib son medicamentos aprobados por la Food and Drug Administration (FDA) de Estados Unidos utilizados en el tratamiento de diversas enfermedades, pues actúan sobre diferentes enzimas biológicas esenciales.
Objetivo. Evaluar los efectos terapéuticos de la monoterapia y de los tres fármacos combinados contra Leishmania infantum.
Materiales y métodos. Los efectos leishmanicidas de los tres fármacos sobre los promastigotes se compararon in vitro en cuanto al recuento de parásitos, la concentración del fármaco que proporcionara una respuesta semimáxima y los cambios ultraestructurales del parásito. Se calculó el índice de concentración inhibitoria de fracciones de fármacos combinados de dos maneras y la actividad de los tres fármacos juntos para determinar el efecto sinérgico.
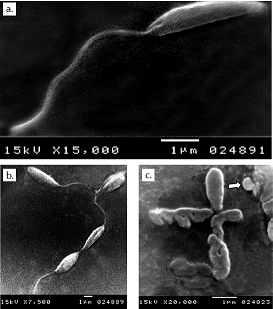
Resultados. La monoterapia con los tres medicamentos fue efectiva, pero la auranofina tuvo el mejor efecto antileishmanicida con un CE50 de 1,5 μM, en tanto que el sorafinib redujo el crecimiento del parásito con un CE50 de 2,5 μM. La microscopía electrónica de barrido de promastigotes de todos los medios tratados mostró una distorsión en la forma, con pérdida de flagelos y formación de ampollas. La acidocalcinosis fue evidente por microscopía electrónica de transmisión con todos los tratamientos, lo que sugiere apoptosis. El tratamiento con lopinavir/ritonavir mostró signos de autofagia. La combinación de dos medicamentos condujo a interacciones aditivas, mientras que la combinación de las tres drogas produjo una acción sinérgica.
Conclusión. Los tres medicamentos usados como monoterapia contra Leishmania spp. fueron efectivos, pero el tratamiento combinado lo fue en mayor medida debido a los efectos aditivos o sinérgicos.
Descargas
Referencias bibliográficas
WHO Expert Committee on the Control of the Leishmaniases and World Health Organization. Control of the leishmaniases: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March, 2010. Geneva: World Health Organization; 2010.
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. https://doi.org/10.1371/journal.pone.0035671
Abou-El-Naga IF. Demographic, socioeconomic and environmental changes affecting the circulation of neglected tropical diseases in Egypt. Asian Pac J Trop Med. 2015;8:881-8. https://doi.org/10.1016/j.apjtm.2015.10.015
Zijlstra EE. PKDL and other dermal lesions in HIV co-infected patients with Leishmaniasis: Review of clinical presentation in relation to immune responses. PLoS Negl Trop Dis. 2014;20:8:e3258. https://doi.org/10.1371/journal.pntd.0003258
Grogl M, Hickman M, Ellis W, Hudson T, Lazo JS, Sharlow ER, et al. Drug discovery algorithm for cutaneous leishmaniasis. Am J Trop Med Hyg. 2013;88:216-21. https://doi.org/10.4269/ajtmh.11-0812
Amato VS, Tuon FF, Bacha HA, Neto VA, Nicodemo AC. Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Trop. 2008;105:1-9. https://doi.org/10.1016/j.actatropica.2007.08.003
Singh N, Kumar M, Singh RK. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012;5:485-97. https://doi.org/10.1016/S1995-7645(12)60084-4
Ekins S, Williams AJ. Finding promiscuous old drugs for new uses. Pharm Res. 2011;28:1785-91. https://doi.org/10.1007/s11095-011-0486-6
Planer JD, Hulverson MA, Arif JA, Ranade RM, Don R, Buckner FS. Synergy testing of FDA approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl Trop Dis. 2014;8:e2977. https://doi.org/10.1371/journal.pntd.0002977
Das P, Alam MN, Paik D, Karmakar K, De T, Chakraborti T. Protease inhibitors in potential drug development for leishmaniasis. Indian J Biochem Biophys. 2013;50:363-76.
Saha AK, Mukherjee T, Bhaduri A. Mechanism of action of amphotericin B on Leishmania donovani promastigotes. Mol Biochem Parasitol. 1986;19:195-200. https://doi.org/10.1016/0166-6851(86)90001-0
Alvar J, Croft S, Olliaro P. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol. 2006;61:223-74. https://doi.org/10.1016/S0065-308X(05)61006-8
Rigobello MP, Scutari G, Boscolo R, Bindoli A. Induction of mitochondrial permeability transition by auranofin, a gold(I)–phosphine derivative. Br J Pharmacol. 2002;136:1162-8. https://doi.org/10.1038/sj.bjp.0704823
Ilari A, Baiocco P, Messori L, Fiorillo A, Boffi A, Gramiccia M, et al. A gold-containing drug against parasitic polyamine metabolism: The X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids. 2012;42:803-11. https://doi.org/10.1007/s00726-011-0997-9
Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023-33. https://doi.org/10.2147/TCRM.S3285
Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63:769-802. https://doi.org/10.2165/00003495-200363080-00004
Klemba M, Goldberg DE. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:183-91. https://doi.org/10.1016/j.molbiopara.2005.05.015
Derouin F, Santillana-Hayat M. Anti-Toxoplasma activities drugs and interactions with pyrimethamine and sulfadiazine in vitro. Antimicrob Agents Chemother. 2000;44:2575-7. https://doi.org/10.1128/AAC.44.9.2575-2577.2000
Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005;49:2983-5. https://doi.org/10.1128/AAC.49.7.2983-2985.2005
Savoia D, Allice T, Tovo PA. Anti-leishmanial activity of HIV protease inhibitors. Int J Antimicrob Agents. 2005;26:92-4. https://doi.org/10.1016/j.ijantimicag.2005.04.003
Santos LO, Vitório BS, Branquinha MH, Pedroso e Silva CM, Santos AL, d’Avila-Levy CM. Nelfinavir is effective in inhibiting the multiplication and aspartic peptidase activity of Leishmania species, including strains obtained from HIV-positive patients. J Antimicrob Chemother. 2013;68:348-53. https://doi.org/10.1093/jac/dks410
Pozio E. Highly active antiretroviral therapy and opportunistic protozoan infections. Parassitologia. 2004;46:83-9.
Trudel N, Garg R, Messier N, Sundar S, Ouellette M, Tremblay MJ. Intracellular survival of Leishmania species that cause visceral leishmaniasis is significantly reduced by HIV-1 protease inhibitors. J Infect Dis. 2008;198:1292-9. https://doi.org/10.1086/592280
Santos LO, Marinho FA, Altoé EF, Vitório BS, Alves CR, Britto C, et al. HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonensis. PLoS One. 2009;4:e4918. https://doi.org/10.1371/journal.pone.0004918
Demarchi IG, Silveira TG, Ferreira IC, Lonardoni MV. Effect of HIV protease inhibitors on New World Leishmania. Parasitol Int. 2012;61:538-44. https://doi.org/10.1016/j.parint.2012.04.006
Sanderson L, Yardley V, Croft SL. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. J Antimicrob Chemother. 2014;69:1888-91. https://doi.org/10.1093/jac/dku069
Cleghorn LA, Woodland A, Collie IT, Torrie LS, Norcross N, Luksch T, et al. Identification of inhibitors of the Leishmania cdc2-related protein kinase CRK3. Chem Med Chem. 2011;6:2214-24. https://doi.org/10.1002/cmdc.201100344
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127-32. https://doi.org/10.1038/nbt1358
Goldberg DE, Siliciano RF, Jacobs WR. Outwitting evolution: Fighting drug-resistant TB, malaria, and HIV. Cell. 2012;148:1271-83. https://doi.org/10.1016/j.cell.2012.02.021
Ménez C, Buyse M, Besnard M. Interaction between miltefosine and amphotericin B: Consequences for their activities towards intestinal epithelial cells and Leishmania donovani promastigotes in vitro. Antimicrob Agents Chemother. 2006;50:3793-800. https://doi.org/10.1128/AAC.00837-06
Seifert K, Munday J, Syeda T. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J Antimicrob Chemother. 2011;66:850-4. https://doi.org/10.1093/jac/dkq542
de Morais-Teixeira E, Gallupo MK, Rodrigues LF, Romanha AJ, Rabello A. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three New World Leishmania species. J Antimicrob Chemother. 2014;69:150-4. https://doi.org/10.1093/jac/dkt318
Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175-96. https://doi.org/10.1093/bmb/lds031
McCartry-Burke C, Bates PA, Dwyer DM. Leishmania donovani: Use of two different commercially available chemically defined media for the continuous in vitro cultivation of promastigotes. Exp Parasitol. 1991;73:385-7. https://doi.org/10.1016/0014-4894(91)90112-A
Garcia LS. Parasite recovery: Culture methods, animal inoculation and xenodiagnosis. In: García LS, editor. Diagnostic Medical Parasitology. 5th edition. Washington, USA: American Society for Microbiology; 2007. p. 927-8.
Fivelman QL, Adagu IS, Warhurst DC. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:4097-102. https://doi.org/10.1128/AAC.48.11.4097-4102.2004
Klainer AS, Betsch CJ. Scanning-beam electron microscopy of selected microorganisms. J Infect Dis. 1970;121:339-43. https://doi.org/10.1093/infdis/121.3.339
Bozzola JJ. Conventional specimen preparation techniques for transmission electron microscopy of cultured cells. Methods Mol Biol. 2007;369:1-18. https://doi.org/10.1007/978-1-59745-294-6_1
Chan YH. Biostatistics 102: Quantitative data- parametric & non-parametric tests. Singapore Med J. 2003;44:391-6.
Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, Nam J, et al. Anti-leishmanial highthroughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis. 2010;4:e675. https://doi.org/10.1371/journal.pntd.0000675
Gazanion E, Vergnes B, Seveno M. In vitro activity of nicotinamide/anti-leishmanial drug combinations. Parasitol Int. 2011;60:19-24. https://doi.org/10.1016/j.parint.2010.09.005
Crowther GJ, Shanmugam D, Carmona SJ, Doyle MA, Hertz-Fowler C, Berriman M, et al. Identification of attractive drug targets in neglected-disease pathogens using an in silico approach. PLoS Negl Trop Dis. 2010;4:e804. https://doi.org/10.1371/journal.pntd.0000804
Valdivieso E, Dagger F, Rascon A. Leishmania mexicana: Identification and characterization of an aspartyl proteinase activity. Exp Parasitol. 2007;116:77-82. https://doi.org/10.1016/j.exppara.2006.10.006
Alves ÉA, de Miranda MG, Borges TK, Magalhães KG, Muniz-Junqueira MI. Anti-HIV drugs, lopinavir/ritonavir and atazanavir, modulate innate immune response triggered by Leishmania in macrophages: The role of NF-κB and PPAR-γ. Int Immunopharmacol. 2015;24:314-24. https://doi.org/10.1016/j.intimp
Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648-57. https://doi.org/10.1111/jcmm.13008
Mesquita JT, Tempone AG, Reimão JQ. Combination therapy with nitazoxanide and amphotericin B, glucantime, miltefosine and sitamaquine against Leishmania infantum intracellular amastigotes. Acta Trop. 2014;130:112-6. https://doi.org/10.1016/j.actatropica.2013.11.003
Butcher EC. Can cell systems biology rescue drug discovery? Nat Rev Drug Discov. 2005;4:461-7. https://doi.org/10.1038/nrd1754
Lewis MG, Da Fonseca S, Chomont N, Palamara AT, Tardugno M, Mai A, et al. Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS. 2011;25:1347-56. https://doi.org/10.1097/QAD.0b013e328347bd77
Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111-26. https://doi.org/10.1128/CMR.19.1.111-126.2006
Olliaro PL. Drug combinations for visceral leishmaniasis. Curr Opin Infect Dis. 2010;23:595-602. https://doi.org/10.1097/QCO.0b013e32833fca9d
Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, et al. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci USA. 2003;100:7977-82. https://doi.org/10.1073/pnas.1337088100
Sharlow ER, Leimgruber S, Murray S, Lira A, Sciotti RJ, Hickman M, et al. Auranofin is an apoptosis-simulating agent with in vitro and in vivo anti-leishmanial activity. ACS Chem Biol. 2014;21:663-72. https://doi.org/10.1021/cb400800q
González-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquère S, et al. Apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091-102. https://doi.org/10.1242/jcs.02447
Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663-9. https://doi.org/10.1016/j.ceb.2004.09.011
Algunos artículos similares:
- Iveth J. González, Las metacaspasas y su rol en la vida y muerte de los parásitos protozoarios humanos , Biomédica: Vol. 29 Núm. 3 (2009)
- Guillermo Terán-Ángel, Vestalia Rodríguez, Rosilved Silva, Olga Zerpa, Henk Schallig, Marian Ulrich, Maira Cabrera, Herramientas no invasivas en Venezuela: comparación entre las pruebas inmunoserológicas DAT, rK26 y rK39 en el diagnóstico de leishmaniasis visceral , Biomédica: Vol. 30 Núm. 1 (2010)
- María Elena Maldonado, Souad Bousserouel, Francine Gossé, Annelise Lobstein, Francis Raul, Implicación de NF-κB y p53 en la expresión de receptores de muerte-TRAIL y apoptosis por procianidinas en células metastásicas humanas SW620 , Biomédica: Vol. 30 Núm. 4 (2010)
- María Teresa Rugeles, Paula A. Velilla, Carlos J. Montoya, Mecanismos de resistencia natural al VIH en seres humanos: un resumen de 10 años de investigación en población colombiana , Biomédica: Vol. 31 Núm. 2 (2011)
- Jairo E. Mateus, Wilfredo Valdivieso, Indira P. Hernández, Fernando Martínez, Edgar Páez, Patricia Escobar, Acumulación celular y efecto anti-Leishmania de la protoporfirina IX exógena y endógena después del tratamiento fotodinámico , Biomédica: Vol. 34 Núm. 4 (2014)
- Jaime E. Castellanos, José I. Neissa, Sigrid J. Camacho, La infección con el virus del dengue induce apoptosis en células del neuroblastoma humano SH-SY5Y , Biomédica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Claudia Viviana Barbosa, Carlos Enrique Muskus, Luz Yaneth Orozco, Adriana Pabón, Efecto mutagénico y genotóxico, y expresión de los genes Rad51C, Xiap, P53 y Nrf2 inducidos por extractos antipalúdicos de plantas recolectadas en el Vaupés medio, Colombia , Biomédica: Vol. 37 Núm. 3 (2017)
- Benny Weiss-Steider, Yolanda Córdova, Itzen Aguiñiga-Sánchez, Edgar Ledesma-Martínez, Vanihamin Domínguez-Meléndez, Edelmiro Santiago-Osorio, El caseinato de sodio y la caseína α inhiben la proliferación de la línea celular mieloide de ratón 32D clone 3 (32Dcl3) mediante el TNF-α , Biomédica: Vol. 39 Núm. 2 (2019)
- Ricardo G. Díaz, Karina A. Salvatierra, Gustavo A. Silva, Enrique J. Deschutter, Fernando J. Bornay-Llinares, Lucrecia Acosta, Primera caracterización molecular de Leishmania infantum en pacientes con leishmaniosis visceral de la Provincia de Misiones, Argentina , Biomédica: Vol. 39 Núm. 2 (2019)
- Karol Liseth Rueda-Concha , Ana Payares-Mercado , Jesús Guerra-Castillo , Jesús Melendrez , Yasmit Arroyo-Munive, Lily Martínez-Abad , Suljey Cochero , Eduar Elías Bejarano , Luis Enrique Paternina, Circulación de Leishmania infantum y Trypanosoma cruzi en perros domésticos de áreas urbanas de Sincelejo, región Caribe de Colombia , Biomédica: Vol. 42 Núm. 4 (2022)
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |

























