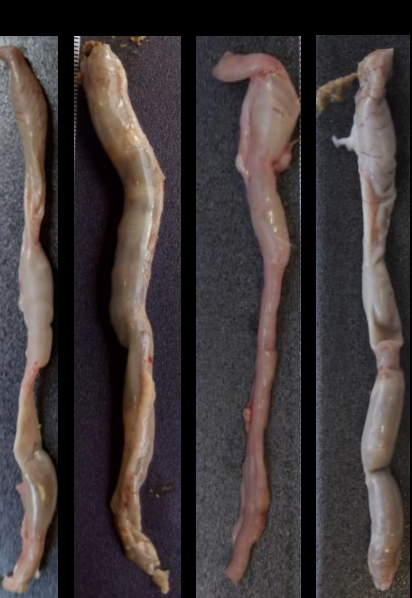
Efectos de la preformulación de Mimosa caesalpiniifolia sobre la barrera intestinal durante la colitis inducida por sulfato de dextrano sódico en ratas Wistar
Resumen
Introducción. Los antiinflamatorios, inmunosupresores e inmunobiológicos se utilizan comúnmente para tratar la enfermedad intestinal inflamatoria. Sin embargo, algunos pacientes no presentan una respuesta adecuada o pierden respuesta efectiva durante el tratamiento. En un estudio reciente, se encontró un potencial efecto antiinflamatorio del extracto hidroalcohólico de Mimosa caesalpiniifolia en la colitis inducida por el ácido trinitrobenceno sulfónico utilizando ratas Wistar.
Objetivo. Evaluar los efectos de la preformulación de M. caesalpiniifolia sobre la barrera intestinal durante la colitis inducida por sulfato de dextrano sódico.
Materiales y métodos. Los extractos de hojas se prepararon con una solución que contenía 70 % de etanol y se secaron con un secador por aspersión Mini B19 de Buchi usando una solución con 20 % de Aerosil®. Treinta y dos ratas Wistar macho se aleatorizaron en cuatro grupos: control basal, colitis sin tratar, control con preformulación (125 mg/kg/día) y colitis tratada con preformulación (125 mg/kg/día). El índice de actividad clínica se registró diariamente y todas las ratas se sacrificaron el noveno día. Los fragmentos de colon se fijaron y se procesaron para análisis histológicos y ultraestructurales. Se recolectaron muestras de heces y se procesaron para el análisis de ácidos grasos de cadena corta.
Resultados. El tratamiento con la preformulación disminuyó la actividad clínica (diarrea sanguinolenta), el infiltrado inflamatorio y las úlceras. La preformulación no reparó la barrera epitelial y no hubo diferencias significativas en el índice de células caliciformes. Se obtuvo una diferencia significativa en los niveles de butirato en las ratas tratadas con la preformulación.
Conclusiones: La preformulación minimizó los síntomas clínicos de colitis e inflamación intestinal pero no minimizó el daño a la barrera intestinal.
Descargas
Referencias bibliográficas
Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13:19-26. https://doi.org/10.5217/ir.2015.13.1.19
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-17. https://doi.org 10.1038/nature10209
Ananthakrishnan AN. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol. 2013;9:367-74. https://doi.org/10.1053/j.gastro.2019.04.016
Chichlows ki M, Hale LP. Bacterial-mucosal interactions in inflammatory bowel disease: an alliance gone bad. Am J Physiol Gastrointest Liver Physiol. 2008;295:1139-49. https://doi.org/10.1152/ajpgi.90516.2008
Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfá G, Nardini V et al. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96-107. https://doi.org/10.1590/1414-431X20143774
Anderson JM, Itallie Van CM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157-65. https://doi.org/10.1016/j.semcdb.2014.08.011
Garud S, Peppercorn MA. Ulcerative colitis: Current treatment strategies and future prospects. Therap Adv Gastroenterol. 2009;2:99-108. https://doi.org/10.1177/1756283X09102329
Carvalho PE. Sabiá (Mimosa caesalpiniifolia). Circular Técnica da Embrapa. 2007;135:1-7.
Aguiar LC, Barros RFM. Plantas medicinais cultivadas em quintais de comunidades rurais no domínio do cerrado piauiense (Município de Demerval Lobão, Piauí, Brasil). Revista Brasileira de Plantas Medicinais. 2012;14:419-34. https://doi.org/10.1590/S1516-05722012000300001
Rakotomalala G, Agard C, Tonnerre P, Tesse A, Derbre S, Michalet S, et al. Extract from Mimosa pigra attenuates chronic experimental pulmonary hypertension. J Ethnopharmacol. 2013;148:106-16. https://doi.org/10.1016/j.jep.2013.03.075
Silva MJ, Endo LH, Dias ALT, Silva GA, Santos MH, Silva MA. Avaliação da atividade antioxidante e antimicrobiana dos extratos e frações orgânicas de Mimosa caesalpiniifolia Benth. (Mimosaceae). Revista de Ciências Farmacêuticas Básica e Aplicada. 2012;33:267-74.
Silva MJ, Carvalho AJ, Rocha CQ, Wilegas W, Silva MA, Gouvêa CM. Ethanolic extract of Mimosa caesalpiniifolia leaves: Chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. S Afr J Bot. 2014;93:64-9. https://doi.org/10.1016/j.sajb.2014.03.011
Silva MJD, de Moura CFG, da Silva VHP, da Silva MA, Vilegas W, Ribeiro DA. Ethanolic extract of Mimosa caesalpiniifolia leaves: modulates chemically induced genotoxicity by cadmium exposure in liver and blood cells of rats. Planta Med. 2014;16:30. https://doi.org/10.1055/s-0034-1394688
Albuquerque UP, Oliveira RF. Is the use-impact on native caatinga species in Brazil reduced by the high species richness of medicinal plants? J Ethnopharmacol. 2017;113:156-70. https://doi.org/10.1016/j.jep.2007.05.025
Silva MJD, Ana SM, Silva N, Dias AT, Vilegas W, Macías A. Bioassay-guided isolation of fungistatic compounds from Mimosa caesalpiniifolia leaves. J Nat Prod. 2019;6:1496-502. https://doi.org/10.1021/acs.jnatprod.8b01025
Silva MD, Vilegas W, Silva MA, Paiotti APR, Pastrelo MM, Ruiz PLM, et al. The antiinflammatory potential of Mimosa caesalpiniifolia following experimental colitis: Role of COX-2 and TNF-Alpha Expression. Drug Res. 2017;67:19. https://doi.org/10.1055/s-0043-119750
Sanchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, Alarcón de la Lastra C. Dietary extra virgin olive oil polyphenols supplementation modulates DSSinduced chronic colitis in mice. J Nutr Biochem. 2013;24:1401-13. https://doi.org/10.1016/j.jnutbio.2012.11.008
Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 ctokines. Clin Exp Immunol. 1998;114:385-91. https://doi.org/10.1046/j.1365-2249.1998.00728.x
Ribeiro WR, Vinolo MAR, Calixto LA. Ferreira CM. Use of gas chromatography to quantify short chain fatty acids in the serum, colonic luminal content and feces of mice. Bioprotocol. 2018;20:e3089. https://doi.org/10.21769/bioprotoc.3089
Silva MJD, Beserra FP, Rodrigues VP, Silva MA, Silva GA, Hiruma-Lima CA, et al. Mimosa caesalpiniifolia (Fabaceae), a medicinal plant from Brazilian cerrado with antioxidant and antiinflammatory properties. Int J Complement Alt Med. 2021;14.
Rejón-Orante JC, Perdomo-Suaréz DP, Rejón-Rodríguez A, Hernández SH, Liévano OEG, Rodríguez DL, et al. Aqueous root extracts from Mimosa albida Humb. & Bonpl. ex Willd display antinociceptive activity in mice. J Ethnopharmacol. 2013;149:522-6. https://doi.org/10.1016/j.jep.2013.07.010
Bendgude RD, Maniyar MG, Kondawar MS, Patil SB, Hirave RV. Anthelmintic activity of leaves of Mimosa pudica. Int J Inst Pharm Life Sci. 2012;2:120-5.
Rivera-Arce E, Chávez-Soto MA, Herrera-Arellano A, Arzate S, Agüero J, Feria-Romero IA, et al. Therapeutic effectiveness of a Mimosa tenuiflora córtex extract in venous leg ulceration treatment. J Ethnopharmacol. 2007;109:523–8. https://doi.org/10.1016/j.jep.2006.08.032
Cheng Z, Zhou H, Yin J, Yu L. ESR estimation of hydroxyl radical scavenging capacity for lipophilic antioxidants. J Agric Food Chem. 2007;55:3325-33. https://doi.org/10.1021/jf0634808
Cohen LJ, Cho JH, Gevers D, Chu H. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology. 2019;156:2174-89. https://doi.org/10.1053/j.gastro.2019.03.017
Rivera-Arce E, Chávez-Soto MA, Herrera-Arellano A, Arzate S, Agüero J, FeriaRomero IA, et al. Therapeutic effectiveness of a Mimosa tenuiflora córtex extract in venous leg ulceration treatment. J Ethnopharmacol. 2007;109:523-8. https://doi.org/10.1016/j.jep.2006.08.032
Octaviano de Souza RS, de Albuquerque UP, Monteiro JM, Cavalcanti de Amorim EL. Jurema-Preta (Mimosa tenuiflora [Willd.] Poir.): a review of its traditional use, phytochemistry and pharmacology. Brazilian Archives of Biology and Technology. 2008;51:93747. https://doi.org/10.1590/S1516-89132008000500010
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. https://doi.org/10.1016/0016-5085(90)90290-H
Benoit C, Jesse DA, Madhu M, Matam VK. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:1-14. https://doi.org/10.1002/0471142735.im1525s104
Nighot, Al-Sadi R, Rawat M, Guo S, Watterson DM, Ma TP. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G988-97. https://doi.org/10.1152/ajpgi.00256.2015
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63-78. https://doi.org/10.1105/tpc.006130
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell. 1997;9:1859-1868. https://doi.org/10.1105/tpc.9.10.1859
Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, et al. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell. 2003;15:2866-84. https://doi.org/10.1105/tpc.016253
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonateethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460-79. https://doi.org/10.1105/tpc.104.025833
Arimura GI, Ozawa R, Kugimiya S, Takabayashi J, Bohlmann J. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-beta-ocimene and transcript accumulation of (E)-beta-ocimene synthase in Lotus japonicus. Plant Physiol. 2004;135:1976-83. https://doi.org/10.1104/pp.104.042929
Arnaud N, Girin T, Sorefan K, Fuentes S, Wood TA, Lawrenson T, et al. Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 2010;24:2127-32. https://doi.org/10.3389/fpls.2011.00107
Mcguckin MA, Eri RD, Das I, Lourie R, Florin TH. Intestinal secretory cell ER stress and inflammation. Biochem Soc Trans. 2011;39:1081-5.
Cummings JH, Rombeau J, Sakata T. Physiological and clinical aspects of short-chain fatty acids. Cambridge: Cambridge University Press; 1995.
Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, et al. Iron modulates butyrate production by a child gut microbiota in vitro. mBio 2015;6: e01453-15. https://doi.org/10.1128/mBio.01453-15
Tedelind S, Westberg F, Kjerrulf M, Vidal A. Antiinflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826-32. https://doi.org/10.3748/wjg.v13.i20.2826
Van Paassen NB, Vincent A, J. Puiman PJ, van der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;13:211-9. https://doi.org/10.1042/BJ20082222
Derechos de autor 2023 Biomédica

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |