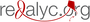Maternal separation during nursing alters basal neuroendocrine levels in juvenile and adult rats
Abstract
Introduction: Work with different animal models including that of maternal separation during nursing has shown that early adverse experiences such as abuse, maternal abandonment and psychosocial stress may favor the development of various psychopathologies. However, several neuroendocrine changes have not been completely described yet. Objective: To establish whether maternal separation during nursing modifies the basal levels of neurohormones such as corticosterone, ACTH, oxytocin and vasopressin in juvenile and adult rats (aged 35 and 90 days, respectively). Materials and methods: Wistar rats were separated from their mothers for two periods of 3 hours per day during the 21 days of nursing. Once these rats had reached 35 and then 90 days of age, blood samples were taken from both the separated and control groups to obtain serum for immunoenzymatic assays and measure the levels of each of the hormones. Results: Concentrations of corticosterone were higher in control adult females in comparison with the rest of the groups and lower in the control adult males. Those of ACTH were higher in the separated young males and females than in the adult groups. Oxytocin levels were significantly higher in the separated adult females in comparison with the other groups and significantly lower in the adult males. With respect to vasopressin, the separated groups had lower concentrations than the young and adult control groups. Conclusions: These results show that the early stress to which rats were submitted produced changes in the basal responses of the hypothalamic-pituitary-adrenal axis, that these responses were distinct in males and females and that they also differed according to age.
Downloads
References
Graham AM, Pfeifer JH, Fisher PA, Carpenter S, Fair DA. Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol Psychiatry . 2015;56:12-22. http://dx.doi.org/10.1111/jcpp.12409
Klanecky AK, Woolman EO, Becker MM. Child abuse exposure, emotion regulation, and drinking refusal self- efficacy: An analysis of problem drinking in college students. Am J Drug Alcohol Abuse . 2015;41:188-96. http://dx.doi.org/10.3109/00952990.2014.998365
Pesonen AK, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DI, et al . Childhood separation experience predicts HPA axis hormonal responses in late adulthood: A natural experiment of World War II, Psychoneuroendocrinology. 2010;35:758-67. http://dx.doi.org/10.1016/j.psyneuen.2009.10.017
Friedman EM, Karlamangla AS, Gruenewald TL, Koretz B, Seeman TE. Early life adversity and adult biological risk profiles. Psychosom Med . 2015;77:176-85. http://dx.doi.org/10.1097/PSY.0000000000000147
Klug H, Bonsall MB. What are the benefits of parental care? The importance of parental effects on developmental rate. Ecol Evol . 2014;4:2330-51. http://dx.doi.org/10.1002/ece3.1083
Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: What can we learn from animal models? Front Neuroendocrinol . 2009;30:497-518. http://dx.doi.org/10.1016/j.yfrne.2009.03.003
Macrì S, Chiarotti F, Würbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav Brain Res. 2008;191: 227-34. http://dx.doi.org/10.1016/j.bbr.2008.03.031
León D, Dueñas Z. Efectos de la separación materna temprana sobre el desempeño en el laberinto en cruz elevado en ratas adultas. Acta Biol Colomb. 2012;17: 129- 42.
Lehmann J, Feldon J. Long-term bio-behavioural effects of maternal separation in the rat: Consistent or confusing? Rev Neurosci. 2000;11:383-408.
Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091-8. http://dx.doi.org/10.1111/j.1460-9568.2007.05522.x
Bautista E, Dueñas Z. Maternal separation during breastfeeding induces changes in the number of cells immunolabeled to GFAP. Psychol Neurosci. 2012;5:207-13. http://dx.doi.org/10.3922/j.psns.2012.2.11
León D, Dueñas Z. Maternal separation during breast-feeding induces gender-dependent changes in anxiety and the GABA-A receptor alpha-subunit in adult Wistar Rats. PLoS One. 2013;8:e68010. http://dx.doi.org/10.1371/journal.pone.0068010
Levay EA, Paolini AG, Govic A, Hazi A, Penman J, Kent S. HPA and sympatho-adrenal activity of adult rats perinatally exposed to maternal mild calorie restriction. Behav Brain Res. 2010;208:202-8. http://dx.doi.org/10.1016/j.bbr.2009.11.033
Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164-74. http://dx.doi.org/10.1016/S0006-3223(00)01084-2
Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, et al . Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. J. Neurosci. 2000;20;3926-35.
Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci . 2010;30:12138-50. http://dx.doi.org /10.1523/JNEUROSCI.3245-10.2010
Shea A, Walsh C, MacMillan HL, Steiner M. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and post-traumatic stress disorder in females. Psychoneuroendocrinology. 2004;30:162-78. http://dx.doi.org/10.1016/j.psyneuen.2004.07.001
Kole MH, Swan L, Fuchs E. The anti-depressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci. 2002;16:807-16. http://dx.doi.org/10.1046/j.1460-9568.2002.02136.x
Meijer OC, Kortekaas R, Oitzl MS, de Kloet ER. Acute rise in corticosterone facilitates 5-HT(1A) receptor-mediated behavioural responses. Eur J Pharmacol. 1998;351:7-14. http://dx.doi.org/10.1016/S0014-2999(98)00289-1
Emanuel RL, Thull DL, Girard DM, Majzoub JA. Developmental expression of corticotropin releasing hormone messenger RNA and peptide in rat hypothalamus. Peptides.1989;10:1165-9.
Riad M, García S, Watkins KC, Jodoin N, Doucet E, Langlois X, et al . Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181-94. http://dx.doi.org/10.1002/(SICI)1096-9861(20000207)417:2<181::AID-CNE4>3.0.CO;2-A
Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: Implications for the pathophysiology of depression. Life Sci.1998;62:1985- 98. http://dx.doi.org/10.1016/S0024-3205(98)00027-7
de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271-81. http://dx.doi.org/10.1016/j.neubiorev.2004.10.008
Sapolsky RM, Meaney MJ. Maturation of the adrenocor- tical stress response: Neuroendocrine control mechanisms and the stress hypo-responsive period. Brain Res. 1986; 396:64-76.
Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol. 2000;85:111S-24. http://dx.doi.org/10.1111/j.1469-445X.2000.tb00014.x
Lonstein JS, Morrell JI. Neuroendocrinology and neurochemistry of maternal behavior and motivation. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Biology. Berlin: Springer-Verlag; 2006. p. 1-51.
Campbell A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol Psychol. 2008;77:1-10. http://dx.doi.org/10.1016/j.biopsycho.2007.09.001
Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858-65. http://dx.doi.org/10.1111/j.1365-2826.2008.01726.x
De Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463- 75. http://dx.doi.org/10.1038/nrn1683
Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: Data convergence from animal and human studies. Biol Psychiatry. 2015;78: 167- 77. http://dx.doi.org/10.1016/j.biopsych.2014.12.003
Scott L, Dinan T. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: Implications for the pathophysiology of depression. Life Sci. 1998;62:1985-98. http://dx.doi.org/10.1016/S0024-3205(98)00027-7
Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Family disruption in childhood and risk of adult depression. Am J Psychiatry . 2003;160:939-46.
Papaioannou A, Gerozissis K, Prokopiou A, Bolaris S , Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal´s response to stress and the vulnerability for depressive behaviour. Behav Brain Res. 2002;129:131-9. http://dx.doi.org/10.1016/S0166-4328(01)00334-5
Park MK, Hoang TA, Belluzzi JD, Leslie FM. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J Neuroendocrinol. 2003;15:289-95. http://dx.doi.org/10.1046/j.1365-2826.2003.01010.x
Duval F, González F, Rabia H. Neurobiología del estrés. Rev Chil Neuro-Psiquiatr. 2010;48:307-18. http://dx.doi.org/10.4067/S0717-92272010000500006
McQuaid R, McInnis O, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neurosci Biobehav Rev. 2014;45:305-22. http://dx.doi.org/10.1016/j.neubiorev.2014.07.005
Uvnäs-Moberg K. Oxytocin linked antistress effects-- the relaxation and growth response. Acta Physiol Scand Suppl . 1997;640:38-42.
Nakase S, Kitayama I, Soya H, Hamanaka K, Nomura J. Increased expression of magnocellular arginin vasopressin mRNA in paraventricular nucleus of stress-induced depression-model rats. Life Sci. 1998;63:23-31. http://dx.doi.org/10.1016/S0024-3205(98)00232-X
Kirschbaum C, Kudielka B, Gaab J, Schommer N, Hellhammer D. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus- pituitary-adrenal axis. Psychosom Med. 1999;61:154-62.
Uhart M, Chong R, Oswald L, Lin P, Wand G. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2 006;31:642-52. http://dx.doi.org/10.1016/j.psyneuen.2006.02.003
Link H, Dayanithi G, Fohr K, Gratzi M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Endocrinology. 1992:130:2183-91. http://dx.doi.org/10.1210/endo.130.4.1312449
Bosch OJ. Maternal aggression in rodents: Brain oxytocin and vasopressin mediate pup defense. Philos Trans R Soc Lond B Biol Sci . 2013;368:20130085. http://dx.doi.org/10.1098/rstb.2013.0085
Dinces SM, Romeo RD, McEwen BS, Tang AC. Enhancing offspring hypothalamic-pituitary-adrenal (HPA) regulation via systematic novelty exposure: The influence of maternal HPA function. Front Behav Neurosci. 2014;8:204. http://dx. doi.org/10.3389/fnbeh.2014.00204
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |