Cytotoxic effect of palladium (II) inclusion compounds in beta-cyclodextrin
Abstract
Introduction: Thiosemicarbazones and palladium (II) complexes have antineoplastic activities with mild side effects, for which they are considered new alternative antineoplastic drugs. However, the IC50 ranges of these complexes vary due to differences in their structure and solubility and their sensitivities for various cellular targets. Beta-cyclodextrin is an additive used to improve the solubility and stability of various drugs for therapeutic use, but the combination of beta-cyclodextrin with palladium (II) complexes and thiosemicarbazones has not been tested yet.
Objective: To study the cytotoxic effect of palladium (II) inclusion complexes in beta-cyclodextrin.
Materials and methods: We tested the cytotoxic activity of palladium complexes combined with beta-cyclodextrin in the breast cancer cell line MCF-7 using a sulforhodamine B assay.
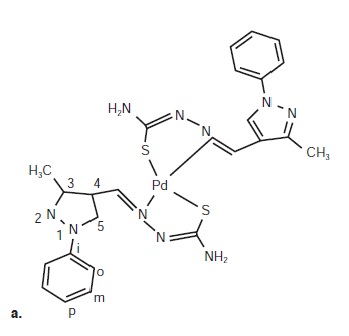
Results: We tested the antiproliferative activity of palladium (II) complexes with and without the ligands MePhPzTSC and Ph2PzTSC and with and without beta-cyclodextrin in MCF-7 cells and compared them to that of cisplatin. All combinations showed antiproliferative activity; however, the activity was greater for the combinations that included beta-cyclodextrin: ([Pd (MePhPzTSC) 2] • ß-CD and [Pd (Ph2PzTSC) 2] • ß-CD), at concentrations of 0.14 and 0.49 μM, respectively. The IC50 for this complex was 5-fold lower than that of the ligand-free combinations (1.4 and 2.9 μM, respectively). The IC50 for free palladium (II) complex was 0.571.24 μM and that for cisplatin was 6.87 μM.
Conclusions: Beta-cyclodextrin significantly enhanced the cytotoxic activities of palladium (II) complexes and thiosemicarbazones probably by improving their solubility and bioavailability. The addition of beta-cyclodextrin is a possible strategy for designing new anticancer drugs.
Downloads
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. http://dx.doi.org/10.3322/caac.20107
Kapdi AR, Fairlamb IJ. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem Soc Rev. 2014;43:4751-77. http://dx.doi.org/ 10.1039/c4cs00063c
Serda M, Kalinowski DS, Rasko N, Potuckova E, Mrozek-Wilczkiewicz A, Musiol R, et al. Exploring the anti-cancer activity of novel thiosemicarbazones generated through the combination of retro-fragments: Dissection of critical structure-activity relationships. PloS One. 2014;9:e110291. http://dx.doi.org/10.1371/journal.pone.0110291
Ari F, Aztopal N, Icsel C, Yilmaz VT, Guney E, Buyukgungor O, et al. Synthesis, structural characterization and cell death-inducing effect of novel palladium(II) and platinum(II) saccharinate complexes with 2-(hydroxymethyl)pyridine and 2-(2-hydroxyethyl)pyridine on cancer cells in vitro. Bioorg Med Chem. 2013;21:6427-34. http://dx.doi.org/10.1016/j.bmc.2013.08.050
Ari F, Cevatemre B, Armutak EI, Aztopal N, Yilmaz VT, Ulukaya E. Apoptosis-inducing effect of a palladium(II) saccharinate complex of terpyridine on human breast cancer cells in vitro and in vivo. Bioorg Med Chem. 2014;22:4948-54. http://dx.doi.org/10.1016/j.bmc.2014.06.039
Padhye S, Afrasiabi Z, Sinn E, Fok J, Mehta K, Rath N. Antitumor metallothiosemicarbazonates: Structure and anti-tumor activity of palladium complex of phenanthrenequinone thiosemicarbazone. Inorg Chem. 2005;44:1154-6. http://dx. doi.org/10.1021/ic048214v
Matesanz AI, Perles J, Souza P. New palladium and platinum complexes with bioactive 3,5-diacetyl-1,2,4-triazol bis(4-cyclohexyl thiosemicarbazone) ligand: Chemistry, antiproliferative activity and preliminary toxicity studies. Dalton Trans. 2012;41:12538-47. http://dx.doi.org/10.1039/C2DT31199B
Prabhakaran R, Kalaivani P, Poornima P, Dallemer F, Huang R, Vijaya Padma V, et al. Synthesis, DNA/protein binding and in vitro cytotoxic studies of new palladium metallothiosemicarbazones. Bioorg Med Chem. 2013;21: 6742-52. http://dx.doi.org/10.1016/j.bmc.2013.08.005
Ulukaya E, Ari F, Dimas K, Ikitimur EI, Guney E, Yilmaz VT. Anti-cancer activity of a novel palladium(II) complex on human breast cancer cells in vitro and in vivo. Eur J Med Chem. 2011;46:4957-63. http://dx.doi.org/10.1016/j.ejmech.2011.07.055
Hernández W, Paz J, Carrasco F, Vaisberg A, Spodine E, Manzur J, et al. Synthesis and characterization of new palladium(II) thiosemicarbazone complexes and their cytotoxic activity against various human tumor cell lines. Bioinorg Chem Appl. 2013;2013:524701. http://dx.doi.org/10. 1155/2013/524701
Jagadeesh M, Rashmi HK, Subba Rao Y, Sreenath Reddy A, Prathima B, Uma Maheswari Devi P, et al. Synthesis and spectroscopic characterization of 3,4-difluoroacetophenone-thiosemicarbazone and its palladium(II) complex: Evaluation of antimicrobial and antitumour activity. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:583-7. http://dx.doi.org/10. 1016/j.saa.2013.06.071
Ramachandran E, Kalaivani P, Prabhakaran R, Rath NP, Brinda S, Poornima P, et al. Synthesis, X-ray crystal structure, DNA binding, antioxidant and cytotoxicity studies of Ni(II) and Pd(II) thiosemicarbazone complexes. Metallomics. 2012;4:218-27. http://dx.doi.org/10.1039/C1MT00143D
Kalaivani P, Prabhakaran R, Dallemer F, Poornima P, Vaishnavi E, Ramachandran E, et al. DNA, protein binding, cytotoxicity, cellular uptake and antibacterial activities of new palladium(II) complexes of thiosemicarbazone ligands: Effects of substitution on biological activity. Metallomics. 2012;4:101-13. http://dx.doi.org/10.1039/c1mt00144b
Sasmal PK, Streu CN, Meggers E. Metal complex catalysis in living biological systems. Chem Commun (Camb). 2013;49: 1581-7. http://dx.doi.org/10.1039/C2CC37832A
Hernández W, Paz J, Vaisberg A, Spodine E, Richter R, Beyer L. Synthesis, characterization, and in vitro cytotoxic activities of benzaldehyde thiosemicarbazone derivatives and their palladium (II) and platinum (II) complexes against various human tumor cell lines. Bioinorg Chem Appl. 2008;2008:690952. http://dx.doi.org/10.1155/2008/690952
Kovala-Demertzi D, Demertzis MA, Miller JR, Papadopoulou C, Dodorou C, Filousis G. Platinum(II) complexes with 2-acetyl pyridine thiosemicarbazone. Synthesis, crystal structure, spectral properties, antimicrobial and antitumour activity. J Inorg Biochem. 2001;86:555-63. http://dx.doi.org/10.1016/S0162-0134(01)00224-0
Kovala-Demertzi D, Varadinova T, Genova P, Souza P, Demertzis MA. Platinum(II) and palladium(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone as alternative antiherpes simplex virus agents. Bioinorg Chem Appl. 2007;2007:56165. http://dx.doi.org/10.1155/2007/56165
Prabhakaran R, Renukadevi SV, Karvembu R, Huang R, Mautz J, Huttner G, et al. Structural and biological studies of mononuclear palladium(II) complexes containing N-substituted thiosemicarbazones. Eur J Med Chem. 2008;43:268-73. http://dx.doi.org/10.1016/j.ejmech.2007. 03.006
Jagadeesh M, Lavanya M, Kalangi SK, Sarala Y, Ramachandraiah C, Varada Reddy A. Spectroscopic characterization, antioxidant and antitumour studies of novel bromo substituted thiosemicarbazone and its copper(II), nickel(II) and palladium(II) complexes. Spectrochim Acta A Mol Biomol Spectrosc. 2015;135:180-4. http://dx.doi.org/10. 1016/j.saa.2014.06.141
Finch RA, Liu M, Grill SP, Rose WC, Loomis R, Vásquez KM, et al. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59:983-91. http://dx.doi.org/10. 1016/S0006-2952(99)00419-0
Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6:409-31. http://dx.doi.org/10.2174/15680090677772 3949
Rosu T, Pahontu E, Pasculescu S, Georgescu R, Stanica N, Curaj A, et al. Synthesis, characterization antibacterial and antiproliferative activity of novel Cu(II) and Pd(II) complexes with 2-hydroxy-8-R-tricyclo[7.3.1.0.(2,7)]tridecane-13-one thiosemicarbazone. Eur J Med Chem. 2010;45:1627-34. http://dx.doi.org/10.1016/j.ejmech.2009.12.015
Tamayo LV, Burgos AE, Brandão PF. Synthesis, characterization, and antimicrobial activity of the ligand 3-methylpyrazole-4-carboxaldehyde thiosemicarbazone and its Pd(II) complex. Phosphorus Sulfur Silicon Relat Elem. 2013;189:52-9. http://dx.doi.org/10.1080/10426507.2013. 777726
Mura P. Analytical techniques for characterization of cyclo-dextrin complexes in the solid state: A review. J Pharm Biomed Anal. 2015;113:226-38. http://dx.doi.org/10.1016/j.jpba.2015.01.058
Burgos AE, Tamayo L, Torrellas-Hidalgo R. Synthesis, characterization and antimicrobial activity of a Pd(II) complex with a 1,3-diphenylpyrazole-4-carboxaldehyde thiosemicarbazone ligand. Rev Udca Actual Divulg Cient. 2014;17:477-86.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107-12. http://dx.doi.org/10.1093/jnci/82.13.1107
Huang R, Wallqvist A, Covell DG. Anticancer metal compounds in NCI’s tumor-screening database: Putative mode of action. Biochem Pharmacol. 2005;69:1009-39. http://dx.doi.org/10.1016/j.bcp.2005.01.001
Mishra L, Singh VK. Synthesis and structural and antifungal studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with new Schiff bases bearing benzimidazoles. Indian J. Chem. 1993;32A:446-9.
Malhota L, Kumar S, Dhindsa KS. Synthesis, charac-terization and microbial activity of Co(II), Ni(II), Cu(II) and Zn(II) complexes of aryloxyacetic acid and hydrazides. Indian J Chem. 1993;32:457-9.
Turanek J, Wang XF, Knotigova P, Koudelka S, Dong LF, Vrublova E, et al. Liposomal formulation of alpha-tocopheryl maleamide: In vitro and in vivo toxicological profile and anticancer effect against spontaneous breast carcinomas in mice. Toxicol Appl Pharmacol. 2009;237:249-57. http://dx. doi.org/10.1016/j.taap.2009.01.027
Matesanz AI, Leitao I, Souza P. Palladium(II) and platinum(II) bis(thiosemicarbazone) complexes of the 2,6-diacetylpyridine series with high cytotoxic activity in cisplatin resistant A2780cisR tumor cells and reduced toxicity. J Inorg Biochem. 2013;125:26-31. http://dx.doi.org/10.1016/j.jinorgbio.2013.04.005
Loftsson T, Duchene D. Cyclodextrins and their pharma-ceutical applications. Int J Pharm. 2007;329:1-11. http://dx. doi.org/10.1016/j.ijpharm.2006.10.044
Codina AV, García A, Leonardi D, Vasconi MD, Di Masso RJ, Lamas MC, et al. Efficacy of albendazole: Beta-cyclodextrin citrate in the parenteral stage of Trichinella spiralis infection. Int J Biol Macromol. 2015;77:203-6. http://dx.doi.org/10.1016/j.ijbiomac.2015.02.049
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743-54. http://dx.doi.org/10. 1021/cr970022c
Kacar O, Adiguzel Z, Yilmaz VT, Cetin Y, Cevatemre B, Arda N, et al. Evaluation of the molecular mechanisms of a palladium(II) saccharinate complex with terpyridine as an anticancer agent. Anticancer Drugs. 2014;25:17-29. http://dx.doi.org/10.1097/CAD.0b013e328364c6ad
Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: Why and how does it occur in biology? Cell Biochem Funct. 2011;29:468-80. http://dx.doi.org/10.1002/cbf.1774
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























