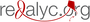STAT3 activation by hypoxia in in vitro models of cervix cancer and endothelial cells
Abstract
Introduction: The biological behavior of cancer cells is influenced by the tumor microenvironment in which they develop. In this context, stressor stimuli such as hypoxia are considered critical for tumor development and therapeutic management. Cellular response to various stimuli is evidenced in the activation of intracellular signaling pathways such as JAK/STAT, which is one of the most important for its effects in differentiation and cell proliferation.
Objective: To evaluate the condition of the JAK/STAT pathway through the expression/activation of the STAT3 protein in cervix cancer cells (HeLa) and endothelial cells (EA.hy926) subjected to hypoxia.
Material and methods: Cell lines were subjected to physical (1% O2) or chemical (deferoxamine, DFO, 100 μM) hypoxia for 2, 6 and 24 hours. Changes in the expression and activation of STAT3, and its subcellular localization by indirect immunofluorescence, were determined by western blot.
Results: Hypoxia was evidenced by the activation and translocation to the nucleus of HIF-1. Neither physical nor chemical hypoxia altered STAT3 expression, but it did affect its activation, as seen in its phosphorylation and translocation to the nucleus in the two models under study.
Conclusions: The present study highlights the importance of hypoxia as a stimulus that modifies the activation of the STAT3 protein in HeLa and EA.hy926 cells, which makes it an important factor in the design of therapeutic strategies against cancer.
Downloads
References
Varmus H. The new era in cancer research. Science. 2006;312:1162-5. http://dx.doi.org/10.1126/science.1126758
Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789-99. http://dx.doi.org/10.1038/nm1087
Caffarel MM, Coleman N. Oncostatin M receptor is a novel therapeutic target in cervical squamous cell carcinoma. J Pathol. 2014;232:386-90. http://dx.doi.org/10.1002/path.4305
Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: Past, present and future. Nat Rev Cancer. 2011;11:749-54. http://dx.doi.org/10.1038/nrc3138
Siemann DW. Tumor microenvironment. Chichester, UK.: John Wiley & Sons, Ltd; 2010. http://dx.doi.org/10.1002/9780470669891
Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319-31. http://dx.doi.org/10.1007/s10555-007-9062-2
Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med. 2007;85:1301-7. http://dx.doi.org/10.1007/s00109-007-0281-3
Liao D, Johnson RS. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281-90. http://dx.doi.org/10.1007/s10555-007-9066-y
Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19-29. http://dx.doi.org/10.1042/BJ20081055
Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol. 2013;86:104-29. http://dx.doi.org/10.1016/j.critrevonc. 2012.09.003
Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J Biol Chem. 2007;282:20059-63.http://dx.doi.org/10.1074/jbc.R700016200
Paukku K, Silvennoinen O. STATs as critical mediators of signal transduction and transcription: Lessons learned from STAT5. Cytokine Growth Factor Rev. 2004;15:435-55. http://dx.doi.org/10.1016/j.cytogfr.2004.09.001
Bowman T, García R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-88. http://dx.doi.org/10.1038/sj.onc.1203527
Takemoto S, Ushijima K, Kawano K, Yamaguchi T, Terada A, Fujiyoshi N, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101:967-72. http://dx.doi.org/10.1038/sj.bjc.6605212
Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266-80.http://dx.doi.org/10.18632/oncotarget.4457
Shukla S, Shishodia G, Mahata S, Hedau S, Pandey A, Bhambhani S, et al. Aberrant expression and constitutive activation of STAT3 in cervical carcinogenesis: Implications in high-risk human papillomavirus infection. Mol Cancer. 2010;9:282. http://dx.doi.org/10.1186/1476-4598-9-282
Grote K, Luchtefeld M, Schieffer B. JANUS under stress--role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol. 2005;43:357-63. http://dx.doi.org/10.1016/j.vph.2005.08.021
Dudley AC, Thomas D, Best J, Jenkins A. A VEGF/JAK2/STAT5 axis may partially mediate endothelial cell tolerance to hypoxia. Biochem J. 2005;390:427-36. http://dx.doi.org/10.1042/BJ20050351
Levy DE, Darnell JE. Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651-62. http://dx.doi.org/10.1038/nrm909
Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122-31. http://dx.doi.org/10.1016/j.tibs.2007.12.002
Pensa S, Regis G, Boselli D, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: Two sides of the same coin? En: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013.
Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V, et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med. 2011;208:1359-66. http://dx.doi.org/10.1084/jem.20110283
Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41-51. http://dx.doi.org/10.1038/nri1995
Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-9. http://dx.doi.org/10.1016/j.cytogfr.2009.11.005
Mauge L, Terme M, Tartour E, Helley D. Control of the adaptive immune response by tumor vasculature. Front Oncol. 2014;4:61. http://dx.doi.org/10.3389/fonc.2014.00061
Pawlus MR, Wang L, Hu C-J. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33:1670-9. http://dx.doi.org/10.1038/onc.2013.115
Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D’angeli L, Bartoli A, et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY). 2010;2:823-42. http://dx.doi.org/10.18632/aging.100232
Wei L-H, Kuo M-L, Chen C-A, Chou C-H, Lai K-B, Lee C-N, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517-27. http://dx.doi.org/10.1038/sj.onc.1206226
Martorell L, Gentile M, Rius J, Rodríguez C, Crespo J, Badimon L, et al. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol Cell Biol. 2009;29:5828-42. http://dx.doi.org/10.1128/MCB.00945-09
Guo M, Song L-P, Jiang Y, Liu W, Yu Y, Chen G-Q. Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxiainducible factor-1alpha independent mechanisms. Apoptosis. 2006;11:67-77. http://dx.doi.org/10.1007/s10495-005-3085-3
Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513-20. http://dx.doi.org/10.1016/j.ccr.2005.05.024
Kaelin WGJ, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. http://dx.doi.org/10.1016/j.molcel.2008.04.009
Zhu P, Ning Y, Yao L, Chen M, Xu C. The proliferation, apoptosis, invasion of endothelial-like epithelial ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res. 2010;29:124. http://dx.doi.org/10.1186/1756-9966-29-124
Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc Natl Acad Sci USA. 2005;102:8150-5.http://dx.doi.org/10.1073/pnas.0501643102
Levy DE. The house that JAK/STAT built. Cytokine Growth Factor Rev.1997;8:81-90. http://dx.doi.org/10.1016/S1359-6101(96)00054-8
Okamoto W, Okamoto I, Arao T, Yanagihara K, Nishio K, Nakagawa K. Differential roles of STAT3 depending on the mechanism of STAT3 activation in gastric cancer cells. Br J Cancer. 2011;105:407-12. http://dx.doi.org/10.1038/bjc.2011.246
Bauer J, Margolis M, Schreiner C, Edgell CJ, Azizkhan J, Lazarowski E, et al. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: Contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992;153:437-49. http://dx.doi.org/10.1002/jcp.1041530302
Schutz A, Roser K, Klitzsch J, Lieder F, Aberger F, Gruber W, et al. Lung adenocarcinomas and lung cancer cell lines show association of MMP-1 expression with STAT3 activation. Transl Oncol. 2015;8:97-105. http://dx.doi.org/10.1016/j.tranon.2015.02.002
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967-75. http://dx.doi.org/10.1038/nrc2540
Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545-51. http://dx.doi.org/10.1016/j.tcb.2008.08.008
Wen Z, Zhong Z, Darnell JE. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241-50.
Yuan Z-L, Guan Y-J, Chatterjee D, Chin YE. STAT3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269-73. http://dx.doi.org/10.1126/science.1105166
Jung JE, Lee HG, Cho IH, Chung DH, Yoon S-H, Yang YM, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296-8. http://dx.doi.org/10.1096/fj.04-3099fje
Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552-60. http://dx.doi.org/10.1038/sj.onc.1208719
Darnell JE. STAT3, HIF-1, glucose addiction and Warburg effect. Aging (Albany NY). 2010;2:890-1. http://dx.doi.org/10.18632/aging.100239
Jung JE, Kim HS, Lee CS, Shin YJ, Kim YN, Kang GH, et al. STAT3 inhibits the degradation of HIF-1alpha by pVHLmediated ubiquitination. Exp Mol Med. 2008;40:479-85.
http://dx.doi.org/10.3858/emm.2008.40.5.479
Hirsila M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 2005;19:1308-10. http://dx.doi.org/10.1096/fj.04-3399fje
Chen H, Guan Y, Yuan G, Zhang Q, Jing N. A perylene derivative regulates HIF-1alpha and Stat3 signaling pathways. Bioorg Med Chem. 2014;22:1496-505. http://dx.doi.org/10.1016/j.bmc.2013.10.018
Demaria M, Poli V. From the nucleus to the mitochondria and back: The odyssey of a multitask STAT3. Cell Cycle. 2011;10:3221-2. http://dx.doi.org/10.4161/cc.10.19.17379
Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270-9.
Yu MO, Park K-J, Park D-H, Chung Y-G, Chi S-G, Kang S-H. Reactive oxygen species production has a critical role in hypoxia-induced Stat3 activation and angiogenesis in human glioblastoma. J Neurooncol. 2015;125:55-63. http://dx.doi.org/10.1007/s11060-015-1889-8
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |