Quercetin ameliorates inflammation in CA1 hippocampal region in aged triple transgenic Alzheimer´s disease mice model.
Abstract
Introduction: Alzheimer’s disease is the most common form of dementia. It is characterized by histopathological hallmarks such as senile plaques and neurofibrillary tangles, as well as a concomitant activation of microglial cells and astrocytes that release pro-inflammatory mediators such as IL-1β, iNOS, and COX-2, leading to neuronal dysfunction and death.
Objective: To evaluate the effect of quercetin on the inflammatory response in the CA1 area of the hippocampus in a 3xTg-AD male and female mice model.
Materials and methods: Animals were injected intraperitoneally with quercetin every 48 hours during three months, and we conducted histological and biochemical studies.
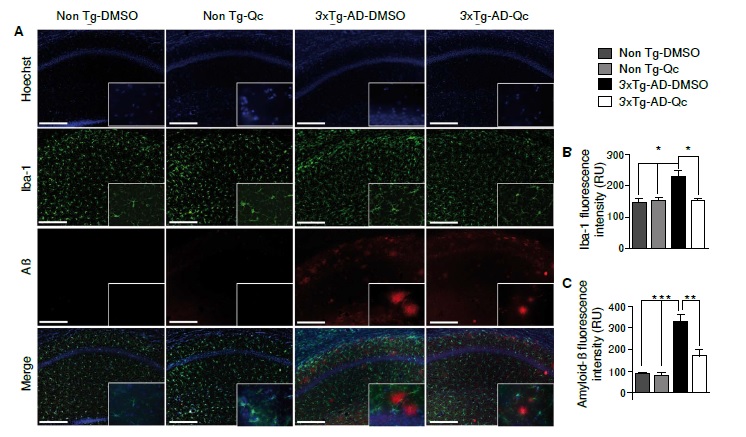
Results: We found that in quercetin-treated 3xTg-AD mice, reactive microglia and fluorescence intensity of Aβ aggregates significantly decreased. GFAP, iNOS, and COX-2 immunoreactivity also decreased and we observed a clear tendency in the reduction of IL-1β in hippocampal lysates.
Conclusion: Our work suggests an anti-inflammatory effect of quercetin in the CA1 hippocampal region of aged triple transgenic Alzheimer’s disease mice.
Downloads
References
Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329-44. https://doi.org/10.1056/NEJMra0909142
Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:1-9 https://doi.org/10.3389/fncel.2014.00112
Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer’s disease. Aging Cell. 2014;13:584-95. https://.doi.org/10.1111/acel.12210
Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354-60.
https://doi.org/10.1523/JNEUROSCI.0616-08.2008
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2008;16:378-85. https://doi.org/10.1038/cdd.2008.172
Carrero I, Gonzalo MR, Martín B, Sanz-Anquela JM, Arévalo-Serrano J, Gonzalo-Ruiz A. Oligomers of beta-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1beta, tumour necrosis factor-alpha, and a nuclear factor kappa-B mechanism in the rat brain. Exp Neurol. 2012;236:215-27. https://doi.org/10.1016/j.expneurol.2012.05.004
Rubio-Pérez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci World J. 2012;2012:1-15. https://doi.org/10.1100/2012/756357
Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605-11.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. https://doi.org/10.1152/physrev.00029.2006
Schopfer F. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem Sci. 2003;28:646-54. https://doi.org/10.1016/j.tibs.2003.10.006
Klegeris A, Walker DG, Mcgeer PL. Activation of macrophages by Alzheimer β amyloid peptide. Biochem Biophys Res Commun. 1994;199:984-91. https://doi.org/10.1006/bbrc.1994.1326
Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117:919-47. https://doi.org/10.1007/s00702-010-0438-z
Zhang D, Hu X, Qian L, Wilson B, Lee C, Flood P, et al. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol Appl Pharmacol. 2009;238:64-70. https://doi.org/10.1016/j.taap.2009.04.015
Nagano T, Kimura SH, Takemura M. Prostaglandin E2 reduces amyloid β-induced phagocytosis in cultured rat microglia. Brain Res. 2010;1323:11-7. https://doi.org/10.1016/j.brainres.2010.01.086
Kanter M, Unsal C, Aktas C, Erboga M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol Ind Health. 2013;32:541-50. https://doi.org/10.1177/0748233713504810
Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ(1-42): Relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269-75. https://doi.org/10.1016/j.jnutbio.2008.03.002
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodríguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134-45. https://doi.org/10.1016/j.neuropharm.2015.01.027
Aronica E, Dickson D, Kress Y, Morrison J, Zukin R. Nonplaque dystrophic dendrites in Alzheimer hippocampus: A new pathological structure revealed by glutamate receptor immunocytochemistry. Neuroscience. 1998;82:979-91. https://doi.org/10.1016/S0306-4522(97)00260-1
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles. Neuron. 2003;39:409-21.
https://doi.org/10.1016/S0896-6273(03)00434-3
Gutiérrez-Vargas J, Castro-Álvarez JF, Velásquez-Carvajal D, Montañez-Velásquez MN, Céspedes-Rubio Á, Cardona-Gómez GP. Rac1 activity changes are associated with neuronal pathology and spatial memory long-term recovery after global cerebral ischemia. Neurochem Int. 2010;57:762-73. https://doi.org/10.1016/j.neuint.2010.08.014
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918-34. https://doi.org/10.1016/j.cell.2010.02.016
von Bernhardi R, Ramírez G, Toro R, Eugenín J. Pro-inflammatory conditions promote neuronal damage mediated by Amyloid Precursor Protein and decrease its phagocytosis and degradation by microglial cells in culture. Neurobiol Dis. 2007;26:153-64. https://doi.org/10.1016/j.nbd.2006.12.006
Wang P, Guan P-P, Wang T, Yu X, Guo J-J, Wang Z-Y. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1β and Aβ between glial and neuron cells. Aging Cell. 2014;13:605-15. https://doi.org/10.1111/acel.12209
Quan Y, Jiang J, Dingledine R. EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem. 2013;288:9293-302. https://doi.org/10.1074/jbc.M113.455816
Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, et al. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest. 2015;125:350-64. https://doi.org/10.1172/JCI77487
Dá Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev. 2016;68:547-62. https://doi.org/10.1016/j.neubiorev.2016.06.014
Heppner FL, Ransohoff RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358-72. https://doi.org/10.1038/nrn3880
Steele ML, Robinson SR. Reactive astrocytes give neurons less support: Implications for Alzheimer’s disease. Neurobiol Aging. 2012;33:423. https://doi.org/10.1016/j.neurobiolaging.2010.09.018
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging. 2014;35:2746-60. https://doi.org/10.1016/j.neurobiolaging.2014.06.004
Kang C-H, Choi YH, Moon S-K, Kim W-J, Kim G-Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013;17:808-13. https://doi.org/10.1016/j.intimp.2013.09.009
Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, et al. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res Bull. 2007;73:55-63. https://doi.org/10.1016/j.brainresbull.2007.01.016
Lu J, Wu D, Zheng Y, Hu B, Zhang Z, Shan Q, et al. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J Pathol. 2010;222:199-212. https://doi.org/10.1002/path.2754
Sung M-S, Lee E-G, Jeon H-S, Chae H-J, Park SJ, Lee YC, et al. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation. 2012;35:1585-94. https://doi.org/10.1007/s10753-012-9473-2
Lavoie S, Chen Y, Dalton TP, Gysin R, Cuénod M, Steullet P, et al. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108:1410-22. https://doi.org/10.1111/j.1471-4159.2009.05908.x
Chen JC, Ho FM, Pei-Dawn LC, Chen C-P, Jeng K-CG, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9-20. https://doi.org/10.1016/j.ejphar.2005.08.005
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, et al. Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. https://doi.org/10.1371/journal.pone.0060921
Zhang X, Hu J, Zhong L, Wang N, Yang L, Liu C-C, et al. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology. 2016;108:179-92. https://doi.org/10.1016/j.neuropharm.2016.04.032
Kong Y, Li K, Fu T, Wan C, Zhang D, Song H, et al. Quercetin ameliorates Aβ toxicity in dosophila AD model by modulating cell cycle-related protein expression. Oncotarget. 2016;7:67716-31. https://doi.org/10.18632/oncotarget.11963
Some similar items:
- Andrés Villegas, Mónica M. Castañeda, Luis Fernando Arias, Beatriz Vieco, Francisco Lopera, Gabriel Bedoya, Evaluation of amyloid-b by the E280A mutation in presenilin gene , Biomedica: Vol. 27 No. 3 (2007)
- Lina María De los Reyes, Ángel Enrique Céspedes, Atorvastatin-meloxicam association inhibits neuroinflammation and attenuates the cellular damage in cerebral ischemia by arterial embolism , Biomedica: Vol. 34 No. 3 (2014)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























