Sodium caseinate and alfa-casein inhibit proliferation of the mouse myeloid cell line 32D clone 3 (32Dcl3) via TNF-α
Abstract
Introduction: Sodium caseinate (CS) and its components (alpha-casein, beta-casein, and kappa-casein) have been shown to inhibit the proliferation of the mouse hematopoietic 32D clone 3 (32Dcl3) cell line and induce its differentiation into macrophages. It is well-known that alpha-casein induces IL-1β production and that this cytokine inhibits the proliferation via the production of tumor necrosis factor alpha (TNF-alpha), but it is not known if CS and the caseins inhibit the proliferation via TNF-alpha production.
Objective: To evaluate if CS and alpha-casein, beta-casein and kappa-casein inhibit the proliferation on 32Dcl3 cell line via TNF-alpha.
Materials and methods: We used different concentrations of CS, alpha-casein, betacasein and kappa-casein in 32Dcl3 cells to evaluate cell proliferation. We assessed cell viability by MTT, induction to apoptosis by flow cytometry, and TNF-alpha synthesis by ELISA. Additionally, we performed anti-TNF-alpha neutralization assays on 32Dcl3 cells treated with CS and alpha-casein and we evaluated proliferation.
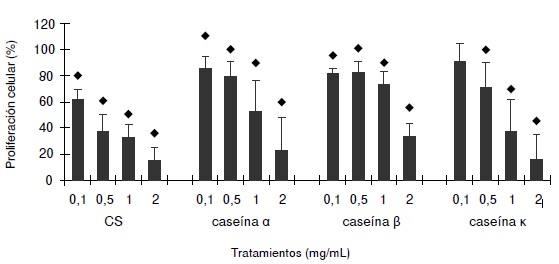
Results: The results showed that CS, alpha-casein, beta-casein, and kappa-casein reduced proliferation of the 32Dcl3 cell line without affecting the viability and that only CS and alpha-casein induced apoptosis and the release of TNF-alpha. The 32Dcl3 cells treated with CS and alpha-casein reestablished their proliferation by using anti-TNF-alpha antibodies.
Conclusion: TNF-alpha was the main responsible for the inhibition of proliferation in 32Dcl3 cells treated with CS or alpha-casein.
Downloads
References
Koletzko B, Agget PJ, Bindels JG, Bung P, Ferré P, Gil A, et al. Growth development and differentiation: A functional food science approach. Br J Nutr. 1998;80:5-45. https://doi.org/10.1079/BJN19980104
Kon S. Milk and milk products in human nutrition. Second edition. Rome: FAO Nutr Stud; 1977.
Walstra P, Jenness R. Dairy chemistry and physics. First edition. New York, USA: Wiley and Sons, 1984.
Warner J. Principios de la tecnología de lácteos. 1a edición. México: AGT; 1976.
Hall C. Drying of milk and products. Second edition. Conneticut, USA: The Avi Pub Company Inc.; 1971.
Okano M, Ohnota H, Sasaki R. Protein deficiency impairs erythropoiesis in rats by reducing serum erythropoietin concentration and the population size of erythroid precursor cells. J Nutr. 1992;122:1376-83. https://doi.org/10.1093/jn/122.7.1376
Wong CW, Seow HF, Liu AH, Husband AJ, Smithers GW, Watson DL. Modulation of immune responses by bovine β-casein. Immunol Cell Biol. 1996;74:323-9. https://doi.org/10.1038/icb.1996.58
Tatsuta M, Lishi H, Baba M, Taniguchi H. Enhanced induction of colon carcinogenesis by azoxymethane in wistar rats fed a low-protein diet. Int J Cancer. 1992;50:108-11. https://doi.org/10.1002/ijc.2910500122
da Silva-Menezes J, de Sousa-Mucida D, Carmona-Cara D, Alvarez-Leite JL, Russo M, Vaz NM, et al. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2003;15:447-55. https://doi.org/10.1093/intimm/dxg043
Kampa M, Loukas S, Hatzoglou A, Martin P, Castanas E. Identification of a novel opioid peptide (Tyr-Val-Pro-Phe-Pro) derived from human alpha S1 casein (alpha S1-casomorphin, and alpha S1-casomorphin amide). Biochem J. 1996;319:903-8. https://doi.org/10.1042/bj3190903
Hatzoglou A, Bakogeorgou E, Hatzoglou C, Martin PM, Castanas E. Antiproliferative and receptor binding properties of α-and β-casomorphins in the T47D human breast cancer cell line. Eur J Pharmacol. 1996;310:217-23. https://doi.org/10.1016/0014-2999(96)00339-1
Ramos-Mandujano G, Weiss-Steider B, Melo B, Córdova Y, Ledesma-Martínez E, Bustos S, et al. Alpha-, beta- and kappa-caseins inhibit the proliferation of the myeloid cell lines 32D cl3 and WEHI-3 and exhibit different differentiation properties. Immunobiology. 2008;213:133-41. https://doi.org/10.1016/j.imbio.2007.07.004
Ramos G, Weiss B, Córdova Y, Hernández J, Zambrano I, Santiago E. Sodium caseinate induces expression and secretion of the murine multipotent myeloid cell line 32D macrophage colonystimulating factor. Arch Med Res. 2004;35:109-13. https://doi.org/10.1016/j.arcmed.2003.11.001
Vordenbaumen S, Saenger T, Braukmann A, Tahan T, Bleck E, Jose J, et al. Human casein alpha s1 induces proinflammatory cytokine expression in monocytic cells by TLR4 signaling. Mol Nutr Food Res. 2016;60:1079-89. https://doi.org/10.1002/mnfr.201500792
Kharbanda S, Nakamura T, Datta R, Sherman ML, Kufe D. Induction of monocytic differentiation by tumor necrosis factor in phorbol ester-resistant KG-1a cells. Cancer Commun. 1990;2:327-32.
Ledesma E, Martínez L, Córdova Y, Rodríguez-Sosa M, Monroy A, Mora L, et al. Interleukin-1 (IL-1β) induces tumor necrosis factor alpha (TNF-α) expression on mouse myeloid multipotent cell line 32D cl3 and inhibits their proliferation. Cytokine. 2004;26:66-72. https://doi.org/10.1016/j.cyto.2003.12.009
Weber C, Aepfelbacher M, Haag H, Ziegler-Heitbrock HW, Weber PC. Tumor necrosis factor induces enhanced responses to platelet-activating factor and differentiation in human monocytic Mono Mac 6 cells. Eur J Immunol. 1993;23:852-9. https://doi.org/10.1002/eji.1830230413
Daneshmandi S, Nourizadeh M, Pourpak Z, Pourfathollah AA. Eliciting Th1 immune response using casein- (Alpha S1) loaded dendritic cells. Iran J Allergy Asthma Immunol. 2017;16:159-68.
Smith JN, Kanwar VS, MacNamara KC. Hematopoietic stem cell regulation by type I and II interferons in the pathogenesis of acquired aplastic anemia. Front Immunol. 2016;29:1-13. https://doi.org/10.3389/fimmu.2016.00330
Tobita K, Kawahara T, Otani H. Bovine beta-casein (1-28), a casein phosphopeptide, enhances proliferation and IL-6 expression of mouse CD19+ cells via Toll-like receptor 4. J Agric Food Chem. 2006;54:8013-7. https://doi.org/10.1021/jf0610864
Megías J, Yáñez A, Moriano S, O’Connor JE, Gozalbo D, Gil ML. Direct Toll-like receptormediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells. 2012;30:1486-95. https://doi.org/10.1002/stem.1110
Otani H, Hata I. Inhibition of proliferative responses of mouse spleen lymphocytes and rabbit Peyer’s patch cells by bovine milk caseins and their digests. J Daily Res. 1995;62:339-48. https://doi.org/10.1017/S0022029900031034
Meisel H. Biochemical properties of peptides encrypted in bovine milk protein. Current Med Chem. 2005;12:1905-19. https://doi.org/10.2174/0929867054546618
Kharbanda S, Nakamura T, Datta R, Sherman ML, Kufe S. Induction of monocytic differentiation by tumor necrosis factor in phorbol ester-resistant KG-1a cells. Cancer Commun. 1990;2:327-32. https://doi.org/10.3727/095535490820874074
Dong QM, Ling C, Chen X, Zhao L. Inhibition of tumor necrosis factor-α enhances apoptosis induced by nuclear factor-κB inhibition in leukemia cells. Oncol Lett. 2015;10:3793-8. https://doi.org/10.3892/ol.2015.3786
Amarante-Mendes GP, Green DR. The regulation of apoptotic cell death. Braz J Med Biol Res. 1999;32:1053-61. https://doi.org/10.1590/S0100-879X1999000900001
Wang JS, Wu-D’Huang DY, Lin WW. TAK1 inhibition-induced RIP1-dependent apoptosis in murine macrophages relies on constitutive TNF-α signaling and ROS production. J Biomed Sci. 2015;22:22-76. https://doi.org/10.1186/s12929-015-0182-7
Morgan JE, Prola A, Mariot V, Pini V, Meng J, Hourde C, et al. Nectroptosis mediates myofibre death in dystrophin-deficient mice. Nat Commun. 2018;9:3655. https://doi.org/10.1038/s41467-018-06057-9
Some similar items:
- Jaime E. Castellanos, José I. Neissa, Sigrid J. Camacho, Dengue virus induces apoptosis in SH-SY5Y human neuroblastoma cells , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Iveth J. González, Metacaspases and their role in the life cycle of human protozoan parasites , Biomedica: Vol. 29 No. 3 (2009)
- Leandro Galvis, Ángel Y. Sánchez, Leonardo F. Jurado, Martha I. Murcia, Tuberculosis associated with tumor necrosis factor-α antagonists, case description and analysis of reported cases in Colombia , Biomedica: Vol. 38 No. 1 (2018)
- Vanihamín Domínguez, Itzen Aguiñiga, Leticia Moreno, Beatriz Torres, Edelmiro Santiago-Osorio, Sodium caseinate increases the number of B lymphocytes in mouse , Biomedica: Vol. 37 No. 4 (2017)
- María Elena Maldonado, Souad Bousserouel, Francine Gossé, Annelise Lobstein, Francis Raul, Implication of NF-κB and p53 in the expression of TRAIL-death receptors and apoptosis by apple procyanidins in human metastatic SW620 cells , Biomedica: Vol. 30 No. 4 (2010)
- María Teresa Rugeles, Paula A. Velilla, Carlos J. Montoya, Mechanisms of human natural resistance to HIV: A summary of ten years of research in the Colombian population , Biomedica: Vol. 31 No. 2 (2011)
- Jorge Machado, Juan Carlos Moncada, Ricardo Pineda, Profile of use of anti tumor necrosis factor in Colombian patients , Biomedica: Vol. 31 No. 2 (2011)
- Alejandra Catalina Vélez, Diana María Castaño, Rubén Darío Gómez, Julio César Orrego, Marcela Moncada, José Luis Franco, Common variable immunodeficiency: Clinical and immunological characterization of patients and homogeneous subgroup definition by means of B lymphocyte subpopulation typing , Biomedica: Vol. 35 No. 1 (2015)
- Claudia Viviana Barbosa, Carlos Enrique Muskus, Luz Yaneth Orozco, Adriana Pabón, Mutagenicity, genotoxicity and gene expression of Rad51C, Xiap, P53 and Nrf2 induced by antimalarial extracts of plants collected from the middle Vaupés region, Colombia , Biomedica: Vol. 37 No. 3 (2017)
- John-Leonardo Torres-Castiblanco, Jorge Alberto Carrillo, Daniel Hincapié-Urrego, Adriana Rojas-Villarraga, Tuberculosis in the era of anti-TNF-alpha therapy: Why does the risk still exist? , Biomedica: Vol. 38 No. 1 (2018)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























