Histopathological study in cardiac tissue of rodents infected with Trypanosoma cruzi, captured in suburbs of Mérida, México
Abstract
Introduction: Trypanosoma cruzi is the causal agent of the American trypanosomiasis, an endemic disease in México. The commensal rodents Mus musculus and Rattus rattus are reservoirs of this parasite, which invades cardiac fibers and develops parasite nests causing various lesions. Histopathological studies in naturally infected rodents are scarce.
Objective: To describe the types and frequencies of microscopic lesions in cardiac tissue of M. musculus and R. rattus infected with T. cruzi captured in Mérida, México.
Materials and methods: The rodents were captured in suburban environments of Mérida. Cardiac tissue was extracted and processed by the paraffin inclusion technique and hematoxylin and eosin stained. The observation was made with a conventional microscope and all the lesions, as well as their degree, were identified.
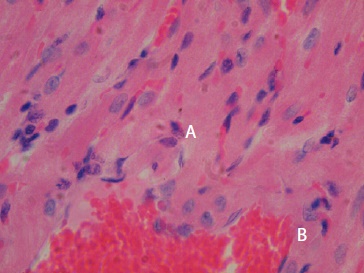
Results: Eight tissue samples of M. musculus and seven of R. rattus were studied. Parasite nests were found in 7/15, specifically 3/8 in M. musculus and 4/7 in R. rattus. The inflammatory infiltrate was the most frequent lesion. Other lesions were: Degeneration of cardiac fibers (8/15), congestion of blood vessels (6/15), and necrosis (5/15).
Discussion: The lesions we observed have been described in experimental animal models and in humans with American trypanosomiasis. The inflammatory infiltrate has been identified as the most significant lesion in humans and reservoirs in the chronic stage of the disease.
Conclusion: The lesions we described are associated with T. cruzi infection, which confirms that the rodents studied are reservoirs of this parasite.
Downloads
References
López-Cancino SA, Tun-Ku E, de la Cruz-Feliz HK, Ibarra-Cerdeña CN, Izeta-Alberdi A, Pech-May A, et al. Landscape ecology of Trypanosoma cruzi in the southern Yucatán Peninsula. Acta Trop. 2015;151:58-72. https://doi.org/10.1016/j.actatropica.2015.07.021
Ramsey JM, Townsend-Peterson A, Carmona-Castro O, Moo-Llanes DA, Nakazawa Y, Butrick M, et al. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Mem Inst Oswaldo Cruz. 2015;110:339-52. https://doi.org/10.1590/0074-02760140404
Moncayo A, Ortiz-Yanine MI. An update on Chagas disease (human American trypanosomiasis). Ann Trop Med Parasitol. 2006;100:663-77.
Leite MF, Moyer MS, Andrews NW. Expression of the mammalian calcium signaling response to Trypanosoma cruzi in Xenopus laevis oocytes. Mol Biochem Parasitol. 1998;92:1-13. https://doi.org/10.1016/S0166-6851(97)00211-9
Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92-100. https://doi.org/10.1016/S1473-3099(01)00065-2
Carrada-Bravo T. Trypanosoma cruzi: historia natural y diagnóstico de la enfermedad de Chagas. Rev Mex Patol Clin. 2004;51:205-19.
Bonney KM, Engman DM. Chagas heart disease pathogenesis: One mechanism or many? Curr Mol Med. 2008;6:510-8. https://doi.org/10.2174/156652408785748004
Postan M, Dvorak JA, McDaniel JP. Studies of Trypanosoma cruzi clones in bred mice. I. A comparison of the course of infection of C3H/HeN mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983;32:497-506. https://doi.org/10.4269/ajtmh.1984.33.236
Andrade LO, Galvão LM, Meirelles MN, Chiari E, Pena SD, Macedo AM. Differential tissue tropism of Trypanosoma cruzi strains: An in vitro study. Mem Inst Oswaldo Cruz. 2010;105:834-7. https://doi.org/10.1590/S0074-02762010000600018
Rozas M, Botto-Mahan C, Coronado X, Ortiz S, Cattan PE, Solari A. Coexistence of Trypanosoma cruzi genotypes in wild and peridomestic mammals in Chile. Am J Trop Med Hyg. 2007;77:643-53.
Rademaker V, Herrera HM, Raffel TR, D’Andrea PS, Freitas TPT, Abreu UG, et al. What is the role of small rodents in the transmission cycle of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida Trypanosomatidae)? A study case in the Brazilian Pantanal. Acta Trop. 2009;111:102-7. https://doi.org/10.1016/j.actatropica.2009.02.006
Brigada AM, Doña R, Caviedes-Vidal E, Moretti E, Basso B. American Tripanosomiasis: A study on the prevalence of Trypanosoma cruzi and Trypanosoma cruzi-like organisms in wild rodents in San Luis province, Argentina. Rev Soc Bras Med Trop. 2010;43:249-53. https://doi.org/10.1590/S0037-86822010000300007
Alemán A, Guerra T, Maikis TJ, Milholland MT, Castro-Arellano I, Forstner MR, et al. The prevalence of Trypanosoma cruzi, the causal agent of Chagas disease, in Texas rodent populations. Ecohealth. 2017;14:130-143. https://doi.org/10.1007/s10393-017-1205-5
Gürtler RE, Cardinal MV. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32-50. https://doi.org/10.1016/j.actatropica.2015.05.029
Zavala-Velázquez J, Barrera-Pérez M, Rodríguez-Félix ME, Guzmán-Marín E, Ruíz-Piña H. Infection by Trypanosoma cruzi in mammals in Yucatán, México: A serological and parasitological study. Rev Inst Med Trop Sao Paulo. 1996;38:289-92.
Panti-May JA, de Andrade RR, Gurubel-González Y, Palomo-Arjona E, Sodá-Tamayo L, Meza-Sulú J, et al. A survey of zoonotic pathogens carried by house mouse and black rat populations in Yucatán, México. Epidemiol Infect. 2017;145:2287-95. https://doi.org/10.1017/S0950268817001352
Panti-May JA, Hernández-Betancourt SF, Torres-Castro MA, Machaín-Williams C, Cigarroa-Toledo N, Sodá L, et al. Population characteristics of human-commensal rodents present in households from Mérida, Yucatán, México. MANTER: Journal of Parasite Biodiversity. 2016;5. https://doi.org/10.13014/K2VD6WCX
Organización Panamericana de la Salud (OPS). Guía para vigilancia, prevención, control y manejo clínico de la Enfermedad de Chagas aguda transmitida por alimentos. Fecha de consulta: 10 de octubre de 2017. Disponible en: http://bvs.panalimentos.org/local/File/Guia_Enfermedad_Chagas_2009esp.pdf
de Diego JA, Palau MT, Gamallo C, Penin P. Relationships between histopathological findings and phylogenetic divergence in Trypanosoma cruzi. Trop Med Int Health. 1998;3:222-33. https://doi.org/10.1111/j.1365-3156.1998.tb00275.x
Acosta-Viana KY, Guzmán-Marín E, Jiménez-Coello M, Torres-León MA, Colín-Flores RF, Ortega-Pacheco A. Cardiac lesions in naturally infected dogs with Trypanosoma cruzi. J Agr Sci Tech. 2011;A1:932-8.
Torres-Castro MA, Hernández-Betancourt SF, Torres-León MA, Puerto FI. Lesiones histológicas asociadas a la posible infección por Trypanosoma cruzi (Chagas, 1909) en corazones de roedores sinantrópicos capturados en Yucatán, México. An Biol. 2016;38:29-35.
Orellana R, Islebe G, Espadas C. Presente, pasado y futuro de los climas de la Península de Yucatán. Naturaleza y sociedad en el área Maya. Pasado, presente y futuro. Primera edición. México, DF: Academia Mexicana de Ciencias, CICY; 2003. p. 37-52.
Flores JS, Espejel I. Tipos de vegetación de la Península de Yucatán. Etnoflora Yucatanense, Fascículo 3. Primera edición. Mérida, México: Universidad Autónoma de Yucatán; 1994. p. 135.
Hernández-Betancourt S, Cimé-Pool J, Medina-Peralta S, González-Villanueva M. Fluctuación poblacional de Ototylomys Phyllotis Merriam, 1901 (Rodentia: Muridae) en una selva mediana subcaducifolia del sur de Yucatán, México. Acta Zool Mex. 2008;24:161-77.
Gaertner DJ, Hallman TM, Hankenson FC, Batchelder MA. Anesthesia and analgesia for laboratory rodents. En: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals. Second edition. London: Elsevier; 2008. p. 239-97.
Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, et al. AVMA Guidelines for the Euthanasia of Animals: 2013 edition. Schaumburg, IL: American Veterinary Medical Association; 2013. p.102.
Armed Forces Institute of Pathology (AFIP). Methods in Histotechnology. Third edition. Washington, D.C.: American Registry of Pathology; 1992. p.279.
Torres-Castro M, Guillermo-Cordero L, Hernández-Betancourt S, Gutiérrez-Ruíz E, Agudelo-Flórez P, Peláez-Sánchez R, et al. First histopathological study in kidneys of rodents naturally infected with Leptospira pathogenic species from Yucatán, México. Asian Pac J Trop Med. 2016;9:145-7. https://doi.org/10.1016/j.apjtm.2016.01.018
Trigo T, Valero E. Patología general veterinaria. Cuarta edición. México, D.F.: Servicios editoriales de la Universidad Autónoma de México; 2004. p. 437.
Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;27:1477-82.
Andrade ZA, Andrade SG, Correa R, Sadigursky M, Ferrans VJ. Myocardial changes in acute Trypanosoma cruzi infection. Ultrastructural evidence of immune damage and the role of microangiopathy. Am J Pathol. 1994;144:1403-11.
Zúñiga C, Vargas R, Vergara U. Evolución de la infección con Trypanosoma cruzi en cepas susceptibles y resistentes de ratones. Arch Med Vet. 2002;34:183-8. https://doi.org/10.4067/S0301-732X2002000200004
Díaz-Limay E, Escalante H, Jara CA. Niveles de parasitemia y alteraciones histopatológicas en Mus musculus BALB/c infectado con Trypanosoma cruzi obtenido de Panstrongylus chinai del Valle Chamán, La Libertad – Perú. Parasitol Latinoam. 2004;59:153-8. https://doi.org/10.4067/S0717-77122004000300011
Marinho CR, Bucci DZ, Dagli ML, Bastos KR, Grisotto MG, Sardinha LR, et al. Pathology affects different organs in two mouse strains chronically infected by a Trypanosoma cruzi clone: A model for genetic studies of Chagas’ disease. Infect Immun. 2004;72:2350-7. https://doi.org/10.1128/IAI.72.4.2350-2357.2004
Roellig DM, Yabsley MJ. Infectivity, pathogenicity, and virulence of Trypanosoma cruzi isolates from sylvatic animals and vectors, and domestic dogs from the United States in ICR strain mice and SD strain rats. Am J Trop Med Hyg. 2010;83:519-22. https://doi.org/10.4269/ajtmh.2010.09-0663
Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velásquez DE, et al. Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am J Pathol. 2011;179:281-8. https://doi.org/10.1016/j.ajpath.2011.03.043
León CM, Montilla M, Vanegas R, Castillo M, Parra E, Ramírez JD. Murine models susceptibility to distinct Trypanosoma cruzi I genotypes infection. Parasitology. 2017;144:512-9. https://doi.org/10.1017/S0031182016001980
Araujo-Carreira JC, Jansen AM, Deane MP, Lenzi HL. Histopathological study of experimental and natural infections by Trypanosoma cruzi in Didelphis marsupialis. Mem Inst Oswaldo Cruz. 1996;91:609-18. https://doi.org/10.1590/S0074-02761996000500012
Pizzi T, Wallace RA, Villagra OR, Muñoz VS, Ortiz ZS, Solari IA. Concordancia de lesiones histológicas en ratones infectados por dos poblaciones de Trypanosoma cruzi de Chile. Rev Med Chile. 2005;133:432-8. https://doi.org/10.4067/S0034-98872005000400006
Rossi MA, Tanowitz HB, Malvestio LM, Celes MR, Campos EC, Blefari V, et al. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanism. PLoS Negl Trop Dis. 2010;4:e674. https://doi.org/10.1371/journal.pntd.0000674
Bazan C, Micucci L, Romina F, Triquel MF, Lo Presti MS, Baez A, et al. Persistence of Trypanosoma cruzi in experimental chagasic cardiomyopathy. Anti-Infective Agents. 2012;10:136-41. https://doi.org/10.2174/2211362611208020136
Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163-72. https://doi.org/10.1016/S0166-6851(99)90035-X
Moreno-Medina E, Valerio-Campos I, Goyenaga-Castro P. Miocarditis y miocardiopatía dilatada por Trypanosoma cruzi: reporte de un caso. Parasitol Latinoam. 2007;62:148-53. https://doi.org/10.4067/S0717-77122007000200008
Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect Dis. 1999;180:480-6. https://doi.org/10.1086/314889
Kierszenbaum F. Mechanisms of pathogenesis in Chagas disease. Acta Parasitol. 2007;52:1-12. https://doi.org/10.2478/s11686-006-0048-y
Cruz L, Vivas A, Montilla M, Hernández C, Flórez C, Parra E, et al. Comparative study of the biological properties of Trypanosoma cruzi I genotypes in a murine experimental model. Infect Genet Evol. 2015;29:110-117. https://doi.org/10.1016/j.meegid.2014.11.012
Jansen AM, Carreira JC, Deane MP. Infection of a mammal by monogenetic insect trypanosomatids (kynetoplastida, trypanosomatidae). Mem Inst Oswaldo Cruz. 1988;3:271-2. https://doi.org/10.1590/S0074-02761988000300001
Tarleton R. Chagas disease: A role for autoimmunity? Trends Parasitol. 2003;19:447-51. https://doi.org/10.1016/j.pt.2003.08.008
Villela-Ribeiro LC, Barbosa AA Jr, Andrade ZA. Pathology of intracardiac nerves in experimental Chagas disease. Mem Inst Oswaldo Cruz. 2002;97:1019-25. https://doi.org/10.1590/S0074-02762002000700016
Rendón DA, Genes CM, Triana O. Lesión celular del miocardio y actividad de la ATPsintasa mitocondrial en ratas infectadas con una cepa colombiana de Trypanosoma cruzi. Biomédica. 2007;27(Sup.1):40-9. https://doi.org/10.7705/biomedica.v27i1.247
Roellig DM, McMillan K, Ellis AE, Vandeberg JL, Champagne DE, Yabsley MJ. Experimental infection of two South American reservoirs with four distinct strains of Trypanosoma cruzi. Parasitology. 2010;137:959-66. https://doi.org/10.1017/S0031182009991995
Teixeira AR, Nascimiento RJ, Sturm NR. Evolution and pathology in Chagas disease – A review. Mem Inst Oswaldo Cruz. 2006;101:463-91. http://dx.doi.org/10.1590/S0074-02762006000500001
Some similar items:
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- María Clara Echeverry, Nubia Catalina Tovar, Guillermo Mora, Presence of antibodies to cardiac neuroreceptors in patients with Chagas disease , Biomedica: Vol. 29 No. 3 (2009)
- Concepción Judith Puerta, Johana María Guevara, Paula Ximena Pavía, Marleny Montilla, Rubén Santiago Nicholls, Edgar Parra, Yuli Katherine Barrera, Evaluation of TcH2AF-R and S35-S36 primers in PCR tests for the detection of Trypanosoma cruzi in mouse cardiac tissue , Biomedica: Vol. 28 No. 4 (2008)
- Elpidia Poveda, Pilar Trujillo, Francisco Ruiz, Elizabeth Lopez, Glucose and insulin levels in Wistar rats submitted to high fat diet and treatment with mimetic leptin peptides , Biomedica: Vol. 28 No. 1 (2008)
- Olga Serrano, Florencio Mendoza, Benny Suárez, Ana Soto, Seroepidemiology of Chagas disease in two rural populations in the municipality of Costa de Oro, at Aragua State, northern Venezuela , Biomedica: Vol. 28 No. 1 (2008)
- Guillermo Mora, María Clara Echeverry, Gustavo Enrique Rey, Myriam Consuelo López, Luisa Fernanda Posada, Fabio Aurelio Rivas, Frequency of Trypanosoma cruzi infection in patients with implanted pacemaker , Biomedica: Vol. 27 No. 4 (2007)
- Concepción Judith Puerta, Paula Ximena Pavia, Marleny Montilla, Carolina Flórez, Giomar Herrera, Juan Manuel Ospina, Fred Manrique, Rubén Santiago Nicholls, The first case of congenital Chagas’ disease analyzed by AP-PCR in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Mario Francisco Guerrero, Elements for the effective evaluation of natural products with possible antihypertensive effects , Biomedica: Vol. 29 No. 4 (2009)
- Lauro Figueroa, Francisco Díaz, Avelardo Camacho, Eliseo Díaz, Rolando Marvin, Activity induced by androsterone and hemisuccinate of androsterone on perfusion pressure and vascular resistance , Biomedica: Vol. 29 No. 4 (2009)
- Rubén Santiago Nicholls, Zulma Milena Cucunubá, Angélica Knudson, Astrid Carolina Flórez, Marleny Montilla, Concepción Judith Puerta, Paula Ximena Pavía, Acute Chagas disease in Colombia: a rarely suspected disease. Report of 10 cases presented during the 2002-2005 period , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























