Assessing the oncolytic potential of rotavirus on mouse myeloma cell line Sp2/0-Ag14
Abstract
Introduction: Cancer is the second leading cause of death in the United States, surpassed only by cardiovascular disease. However, cancer has now overtaken cardiovascular disease as the main cause of death in 12 countries in Western Europe. The burden of cancer is posing a major challenge to health care systems worldwide and demanding improvements in methods for cancer prevention, diagnosis, and treatment. Alternative and complementary strategies for orthodox surgery, radiotherapy, and chemotherapy need to be developed.
Objective: To determine the oncolytic potential of tumor cell-adapted rotavirus in terms of their ability to infect and lysate murine myeloma Sp2/0-Ag14 cells.
Materials and methods: We inoculated rotaviruses Wt1-5, WWM, TRUYO, ECwt-O, and WTEW in Sp2/0-Ag14 cells and we examined their infectious effects by immunocytochemistry, immunofluorescence, flow cytometry, and DNA fragmentation assays.
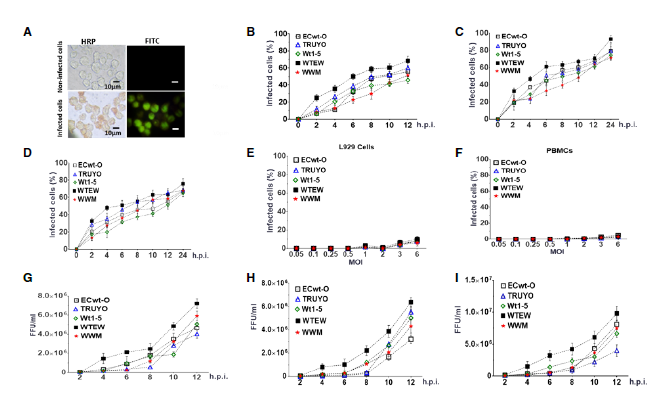
Results: Rotavirus infection involved the participation of some heat shock proteins, of protein disulfide isomerase (PDI), and integrin β3. We detected the accumulation of viral antigens within the virus-inoculated cells and in the culture medium in all the rotavirus isolates examined. The rotavirus-induced cell death mechanism in Sp2/0-Ag14 cells involved changes in cell membrane permeability, chromatin condensation, and DNA fragmentation, which were compatible with cytotoxicity and apoptosis.
Conclusions: The ability of the rotavirus isolates Wt1-5, WWM, TRUYO, ECwt-O, and WTEW to infect and cause cell death of Sp2/0-Ag14 cells through mechanisms that are compatible with virus-induced apoptosis makes them potential candidates as oncolytic agents.
Downloads
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. https://doi.org/10.3322/caac.21387
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur Heart J. 2016;37:3232-45. https://doi.org/10.1093/eurheartj/ehw334
Guo W, Chen W, Yu W, Huang W, Deng W. Small interfering RNA-based molecular therapy of cancers. Chin J Cancer. 2013;32:488-93. https://doi.org/10.5732/cjc.012.10280
Garzón R, Marcucci G, Croce CM. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775-89. https://doi.org/10.1038/nrd3179
Morton SW, Lee MJ, Deng ZJ, Dreaden EC, Siouve E, Shopsowitz KE, et al. A nanoparticlebased combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal. 2014;7:325-44. https://doi.org/10.1126/scisignal.2005261
Atherton MJ, Lichty BD. Evolution of oncolytic viruses: Novel strategies for cancer treatment. Immunotherapy. 2013;5:1191-206. https://doi.org/10.2217/imt.13.123
Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220-8. https://doi.org/10.1038/nrm2858
McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15-26. https://doi.org/10.1016/j.ccell.2014.12.001
Merajver S, Phadke S, Soellner M. Conquering the challenges of genotypic and phenotypic tumor heterogeneity to realize the promise of personalized cancer therapy: The role of academia. Trans Am Clin Climatol Assoc. 2017;128:169-79.
Berghella AM, Contasta I, Lattanzio R, Di Gregorio G, Campitelli I, Silvino M, et al. The role of gender-specific cytokine pathways as drug targets and gender-specific biomarkers in personalized cancer therapy. Curr Drug Targets. 2017;18:485-95. https://doi.org/10.2174/1389450117666160630173647
Hainaut P, Plymoth A. Targeting the hallmarks of cancer: Towards a rational approach to next-generation cancer therapy. Curr Opin Oncol. 2013;25:50-1. https://doi.org/10.1097/CCO.0b013e32835b651e
Singh PK, Doley J, Kumar GR, Sahoo AP, Tiwari AK. Oncolytic viruses and their specific targeting to tumour cells. Indian J Med Res. 2012;136:571-84.
Howells A, Marelli G, Lemoine NR, Wang Y. Oncolytic viruses-interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. 2017;7:195-9. https://doi.org/10.3389/fonc.2017.00195
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642-62. https://doi.org/10.1038/nrd4663
Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3:745-50. https://doi.org/10.1038/35087061
Garant KA, Shmulevitz M, Pan L, Daigle RM, Ahn DG, Gujar SA, et al. Oncolytic reovirus induces intracellular redistribution of Ras to promote apoptosis and progeny virus release. Oncogene. 2016;35:771-82. https://doi.org/10.1038/onc.2015.136
Kohlhapp FJ, Kaufman HL. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22:1048-54. https://doi.org/10.1158/1078-0432.CCR-15-2667
Ebrahimi S, Ghorbani E, Khazaei M, Avan A, Ryzhikov M, Azadmanesh K, et al. Interferonmediated tumor resistance to oncolytic virotherapy. J Cell Biochem. 2017;118:1994-9. https://doi.org/10.1002/jcb.25917
Vaha-Koskela M, Hinkkanen A. Tumor restrictions to oncolytic virus. Biomedicines. 2014;2:163-94. https://doi.org/10.3390/biomedicines2020163
Okamoto J, Mikami I, Tominaga Y, Kuchenbecker KM, Lin YC, Bravo DT, et al. Inhibition of Hsp90 leads to cell cycle arrest and apoptosis in human malignant pleural mesothelioma. J Thorac Oncol. 2008;3:1089-95. https://doi.org/10.1097/JTO.0b013e3181839693
Truman AW, Kristjansdottir K, Wolfgeher D, Hasin N, Polier S, Zhang H, et al. CDKdependent Hsp70 phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151:1308-18. https://doi.org/10.1016/j.cell.2012.10.051
Diehl JA, Yang W, Rimerman RA, Xiao H, Emili A. Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol Cell Biol. 2003;23:1764-74. https://doi.org/10.1128/MCB.23.5.1764-1774.2003
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: Essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743-61. https://doi.org/.1111/j.1582-4934.2008.00273.x
Basu S, Srivastava PK. Heat shock proteins: The fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443-51. https://doi.org/10.1379/1466-1268(2000)005<0443:HSPTFO>2.0.CO;2
Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193:5765-71. https://doi.org/10.4049/jimmunol.1401417
Lianos GD, Alexiou GA, Mangano A, Rausei S, Boni L, Dionigi G, et al. The role of heat shock proteins in cancer. Cancer Lett. 2015;360:114-8. https://doi.org/10.1016/j.canlet.2015.02.026
Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181-8. https://doi.org/10.1093/carcin/bgt111
Lee E, Lee DH. Emerging roles of protein disulfide isomerase in cancer. BMB Rep. 2017;50:401-10. https://doi.org/10.1093/carcin/bgt111
Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-targeted cancer therapy. Drug Dev Res. 2008;69:329-39. https://doi.org/10.1002/ddr.20265
Mangurten AB, Brader KR, Loos BM, Lee E, Quiroga AI, Bathori J, et al. Hsp70 and Hsc70 are preferentially expressed in differentiated epithelial cells in normal human endometrium and ectocervix. Cell Stress Chaperones. 1997;2:168-74. https://doi.org/10.1379/1466-1268(1997)002<0168:hahape>2.3.co;2
Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168-73.
Calderwood SK, Gong J. Heat shock proteins promote cancer: It’s a protection racket. Trends Biochem Sci. 2016;41:311-23. https://doi.org/10.1016/j.tibs.2016.01.003
Guerrero CA, Bouyssounade D, Zarate S, Isa P, López T, Espinosa R, et al. Heat shock cognate protein 70 is involved in rotavirus cell entry. J Virol. 2002;76:4096-102. https://doi.org/10.1128/JVI.76.8.4096-4102.2002
Calderón MN, Guerrero CA, Acosta O, López S, Arias CF. Inhibiting rotavirus infection by membrane-impermeant thiol/disulfide exchange blockers and antibodies against protein disulfide isomerase. Intervirology. 2012;55:451-64. https://doi.org/10.1159/000335262
Guerrero CA, Méndez E, Zarate S, Isa P, López S, Arias CF. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci USA. 2000;97:14644-9. https://doi.org/10.1073/pnas.250299897
Guerrero CA, Guerrero RA, Silva E, Acosta O, Barreto E. Experimental adaptation of rotaviruses to tumor cell lines. PLoS One. 2016;11:e0147666. https://doi.org/10.1371/journal.pone.0147666
Calderón MN, Guzmán F, Acosta O, Guerrero CA. Rotavirus VP4 and VP7-derived synthetic peptides as potential substrates of protein disulfide isomerase lead to inhibition of rotavirus infection. Int J Pept Res Ther. 2012;18:373-82 . https://doi.org/10.1007/s10989-012-9314-z
Prieto I, Hervas-Stubbs S, García-Granero M, Berasain C, Riezu-Boj JI, Lasarte JJ, et al. Simple strategy to induce antibodies of distinct specificity: Application to the mapping of gp120 and inhibition of HIV-1 infectivity. Eur J Immunol. 1995;25:877-83. https://doi.org/10.1002/eji.1830250403
Arnold M, Patton JT, McDonald SM. Culturing, storage, and quantification of rotaviruses. Curr Protoc Microbiol. 2009. https://doi.org/10.1002/9780471729259.mc15c03s15
Moreno LY, Guerrero CA, Acosta O. Protein disulfide isomerase and heat shock cognate protein 70 interactions with rotavirus structural proteins using their purified recombinant versions. Revista Colombiana de Biotecnología. 2016;18:33-48. https://doi.org/10.15446/rev.colomb.biote.v18n1.57714
Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: What have we learned from the deficient mouse model? Mutat Res. 2000;460:1-15. https://doi.org/10.1016/S0921-8777(00)00016-1
Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch Toxicol. 2013;87:19-48. https://doi.org/10.1007/s00204-012-0918-z
Samanta S, Tamura S, Dubeau L, Mhawech-Fauceglia P, Miyagi Y, Kato H, et al. Expression of protein disulfide isomerase family members correlates with tumor progression and patient survival in ovarian cancer. Oncotarget. 2017;8:103543-56. https://doi.org/10.18632/oncotarget.21569
Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci USA. 2009;106:10666-71. https://doi.org/10.1073/pnas.0903035106
Havaki S, Kouloukoussa M, Amawi K, Drosos Y, Arvanitis LD, Goutas N, et al. Altered expression pattern of integrin alphavbeta3 correlates with actin cytoskeleton in primary cultures of human breast cancer. Cancer Cell Int. 2007;7:16-22. https://doi.org/10.1186/1475-2867-7-16
Bai SY, Xu N, Chen C, Song YL, Hu J, Bai CX. Integrin alphavbeta5 as a biomarker for the assessment of non-small cell lung cancer metastasis and overall survival. Clin Respir J. 2015;9:457-67. https://doi.org/10.1111/crj.12163
Gualtero DF, Guzmán F, Acosta O, Guerrero CA. Amino acid domains 280-297 of VP6 and 531-554 of VP4 are implicated in heat shock cognate protein hsc70-mediated rotavirus infection. Arch Virol. 2007;152:2183-96. https://doi.org/10.1007/s00705-007-1055-5
Kim MY, Oglesbee M. Virus-heat shock protein interaction and a novel axis for innate ntiviral immunity. Cells. 2012;1:646-66. https://doi.org/10.3390/cells1030646
Khandjian EW, Turler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983;3:1-8. https://doi.org/10.1128/MCB.3.1.1
Wu BJ, Hurst HC, Jones NC, Morimoto RI. The E1A 13S product of adenovirus 5 activates transcription of the cellular human HSP70 gene. Mol Cell Biol. 1986;6:2994-9. https://doi.org 10.1128/MCB.6.8.2994
Phillips B, Abravaya K, Morimoto RI. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. J Virol. 1991;65:5680-92.
Kaminskyy V, Zhivotovsky B. To kill or be killed: How viruses interact with the cell death machinery. J Intern Med. 2010;267:473-82. https://doi.org/10.1111/j.1365-2796.2010.02222.x
Bautista D, Rodríguez LS, Franco MA, Ángel J, Barreto A. Caco-2 cells infected with rotavirus release extracellular vesicles that express markers of apoptotic bodies and exosomes. Cell Stress Chaperones. 2015;20:697-708. https://doi.org/10.1007/s12192-015-0597-9
Pérez JF, Chemello ME, Liprandi F, Ruiz MC, Michelangeli F. Oncosis in MA104 cells is induced by rotavirus infection through an increase in intracellular Ca2+ concentration. Virology. 1998;252:17-27. https://doi.org/10.1006/viro.1998.9433
Bagchi P, Dutta D, Chattopadhyay S, Mukherjee A, Halder UC, Sarkar S, et al. Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection. J Virol. 2010;84:6834-45. https://doi.org/10.1128/JVI.00225-10
Zarate S, Romero P, Espinosa R, Arias CF, López S. VP7 mediates the interaction of rotaviruses with integrin alphavbeta3 through a novel integrin-binding site. J Virol. 2004;78:10839-47. https://doi.org/10.1128/JVI.78.20.10839-10847.2004
Calderón MN, Guerrero CA, Domínguez Y, Garzón E, Barreto SM, Acosta O. Interacción de rotavirus con la proteína disulfuro-isomerasa in vitro y en sistemas celulares. Biomédica. 2011;31:70-81. https://doi.org/10.7705/biomedica.v31i1.337
Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. https://doi.org/10.1371/journal.pone.0062876
Zhang S, Hu Y, Huang Y, Xu H, Wu G, Dai H. Heat shock protein 27 promotes cell proliferation through activator protein-1 in lung cancer. Oncol Lett. 2015;9:2572-6. https://doi.org/10.3892/ol.2015.3073
Isa P, Sánchez-Alemán MA, López S, Arias CF. Dissecting the role of integrin subunits alpha 2 and beta 3 in rotavirus cell entry by RNA silencing. Virus Res. 2009;145:251-9. https://doi.org/10.1016/j.virusres.2009.07.013
Dutta D, Chattopadhyay S, Bagchi P, Halder UC, Nandi S, Mukherjee A, et al. Active participation of cellular chaperone Hsp90 in regulating the function of rotavirus nonstructural protein 3 (NSP3). J Biol Chem. 2011;286:20065-77. https://doi.org/10.1074/jbc.M111.231878
Dutta D, Bagchi P, Chatterjee A, Nayak MK, Mukherjee A, Chattopadhyay S, et al. The molecular chaperone heat shock protein-90 positively regulates rotavirus infectionx. Virology. 2009;391:325-33. https://doi.org/10.1016/j.virol.2009.06.044
Some similar items:
- Raquel Villamizar, Dioselina Peláez-Carvajal , Luis Felipe Acero , un Identification of enteric viruses from raw water using fluoro-immuno-magnetic separation coupled to RT-PCR , Biomedica: Vol. 41 No. 4 (2021)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























