In vitro susceptibility to benznidazole, nifurtimox and posaconazole of Trypanosoma cruzi isolates from Paraguay
Abstract
Introduction: Trypanosoma cruzi, the causative agent of Chagas disease, shows substantial phenotypic and genotypic heterogeneity, which can influence the epidemiological and clinical variations of the disease and the sensitivity to the drugs used in the treatment.
Objective: To assess the in vitro susceptibility to benznidazole, nifurtimox, and posaconazole of 40 cloned strains of T. cruzi isolated in Paraguay belonging to different genotypes, hosts, and localities.
Materials and methods: We incubated the parasites in their epimastigote stage in LIT culture medium with different concentrations of each drug in triplicate assays. The degree of susceptibility was estimated by the inhibitory concentrations of 50 and 90% (IC50 and IC90) to obtain the average values and the standard deviation for each strain and drug. We determined the statistical significance between groups by analysis of variances with the Wilcoxon/Kruskal-Wallis non-parametric test and values of p<0.05.
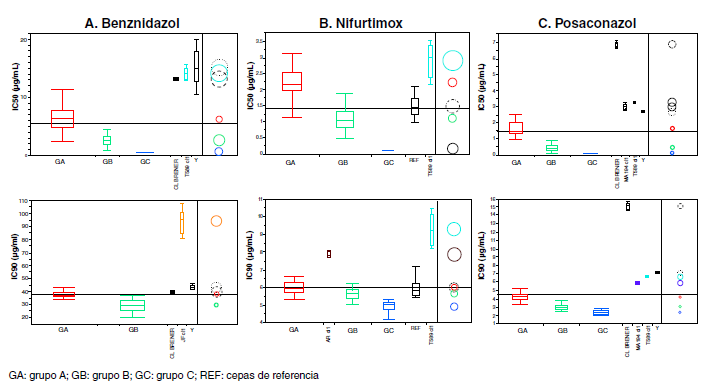
Results: A wide range of drug response was observed. Two groups of parasites (A and B) were identified as having significant differences in susceptibility to benznidazole (p<0.0001), and three groups (A, B, C) to nifurtimox and posaconazole (p<0.0001).
Conclusions: Overall, the isolates were more susceptible to nifurtimox than benznidazole and posaconazole. Such differences highlight the heterogeneity of T. cruzi populations circulating in Paraguay, an aspect to consider in the treatment and follow up of patients.
Downloads
References
Stanaway JD, Roth G. The burden of Chagas disease: Estimates and challenges. Glob Heart. 2015;10:139-44. https://doi.org/10.1016/j.gheart.2015.06.001
World Health Organization (WHO). Integrating neglected tropical diseases into global health and development. Fourth WHO report on neglected tropical diseases, 2017. Fecha de consulta: 20 de mayo del 2019. Disponible en: https://www.who.int/neglected_diseases/resources/9789241565448/en/
Herricks JR, Hotez PJ, Wanga V, Coffeng LE, Haagsma JA, Basáñez MG, et al. The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl Trop Dis. 2017;11:e0005424. https://doi.org/10.1371/journal.pntd.0005424
Buccheri R, Kassab MJ, Freitas VL, Silva SC, Bezerra RC, Khoury Z, et al. Chagasic meningoencephalitis in an HIV infected patient with moderate immunosuppression: Prolonged survival and challenges in the HAART era. Rev Inst Med Trop Sao Paulo. 2015;57:531-5. https://doi.org/10.1590/S0036-46652015000600014
Gray EB, La Hoz RM, Green JS, Vikram HR, Benedict T, Rivera H, et al. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012-2016. Transpl Infect Dis. 2018;20:e12996. https://doi.org/10.1111/tid.12996
Kaushal M, Shabani S, Cochran EJ, Samra H, Zwagerman NT, Kaushal M. Cerebral trypanosomiasis in an immunocompromised patient: Case report and review of the literature. World Neurosurg. 2019;pii:S1878-875031537-2. https://doi.org/10.1016/j.wneu.2019.05.260
Pereiro AC. Guidelines for the diagnosis and treatment of Chagas disease. Lancet.2019;393:1486-7. https://doi.org/10.1016/S0140-6736(19)30288-0
Sangenito LS, da Silva Santos V, d’Avila-Levy CM, Branquinha MH, Souza Dos Santos AL, de Oliveira SSC. Leishmaniasis and Chagas disease - neglected tropical diseases: Treatment updates. Curr Top Med Chem. 2019;19:174-7. https://doi.org/10.2174/156802661903190328155136
Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755-9. https://doi.org/10.1016/0035-9203(87)90020-4
Luna KP, Hernández IP, Rueda CM, Zorro MM, Croft SL, Escobar P. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole. Biomédica. 2009;29:448-55. https://doi.org/10.7705/biomedica.v29i3.15
Mejía-Jaramillo AM, Fernández GJ, Montilla M, Nicholls RS, Triana-Chávez O. Trypanosoma cruzi strains resistant to benznidazole occurring in Colombia. Biomédica. 2012;32:196-205. https://doi.org/10.1186/1756-3305-4-169
Dos Santos FM, Caldas S, de Assis Cáu SB, Crepalde GP, de Lana M, Machado-Coelho GL, et al. Trypanosoma cruzi: Induction of benznidazole resistance in vivo and its modulation by in vitro culturing and mice infection. Exp Parasitol. 2008;120:385-90. https://doi.org/10.1016/j.exppara.2008.09.007
Mejía AM, Hall BS, Taylor MC, Gómez-Palacio A, Wilkinson SR, Triana-Chávez O, et al. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J Infect Dis. 2012;206:220-8. https://doi.org/10.1093/infdis/jis331
Urbina JA, Payares G, Contreras LM, Liendo A, Sanoja C, Molina J, et al. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: In vitro and in vivo studies. Antimicrob Agents Chemother. 1998;42:1771-7. https://doi.org/10.1128/AAC.42.7.1771
Urbina JA, Payares G, Sanoja C, Lira R, Romanha AJ. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int J Antimicrob Agents. 2003;21:27-38. https://doi.org/10.1016/s0924-8579(02)00273-x
Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;37020:1899-908. https://doi.org/10.1056/NEJMoa1313122
Morillo CA, Waskin H, Sosa-Estani S, Del Carmen Bangher M, Cuneo C, Milesi R, et al. STOP-CHAGAS Investigators. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: The STOP-CHAGAS trial. J Am Coll Cardiol. 2017;69:939-47. https://doi.org/10.1016/j.jacc.2016.12.023
Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, et al. The revised Trypanosoma cruzisubspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240-53. https://doi.org/10.1016/j.meegid.2011.12.009
Burgos JM, Begher S, Silva HM, Bisio M, Duffy T, Levin MJ, et al. Molecular identification of Trypanosoma cruzi I tropism for central nervous system in Chagas reactivation due to AIDS. Am J Trop Med Hyg. 2008;78:294-7.
Llewellyn MS, Rivett-Carnac JB, Fitzpatrick S, Lewis MD, Yeo M, Gaunt MW, et al. Extraordinary Trypanosoma cruzi diversity within single mammalian reservoir hosts implies a mechanism of diversifying selection. Int J Parasitol. 2011;41:609-14. https://doi.org/10.1016/j.ijpara.2010.12.004
Bontempi IA, Bizai ML, Ortiz S, Manattini S, Fabbro D, Solari A, et al. Simple methodology to directly genotype Trypanosoma cruzi discrete typing units in single and mixed infections from human blood samples. Infect Genet Evol. 2016;43:123-9. https://doi.org/10.1016/j.meegid.2016.05.026
Chapman MD, Baggaley RC, Godfrey-Fausset PF, Malpas TJ, White G, Canese J, et al. Trypanosoma cruzi from the Paraguayan Chaco: Isoenzyme profiles of strains isolated at Makthlawaiya. J Protozool. 1984;31:482-6. https://doi.org/10.1111/j.1550-7408.1984.tb02999.x
Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV, et al. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40:1599-607. https://doi.org/10.1016/j.ijpara.2010.06.006
del Puerto F, Sánchez Z, Nara E, Meza G, Paredes B, Ferreira E, et al. Trypanosoma cruzi lineages detected in congenitally infected infants and Triatoma infestans from the same disease-endemic region under entomologic surveillance in Paraguay. Am J Trop Med Hyg. 2010;82:386-90. https://doi.org/10.4269/ajtmh.2010.09-0006
Sánchez Z, Russomando G, Chena L, Nara E, Cardozo E, Paredes B, et al. Triatoma sordida, indicadores de adaptación y transmisión de Trypanosoma cruzi en intradomicilio del Chaco Paraguayo. Mem Inst Investig Cienc Salud. 2016;14:96-101. https://doi.org/10.18004/Mem.iics/1812-9528/2016.014(03)96-101
Acosta N, Miret J, López E, Schinini A. First report of Sapajus cay naturally infected by Trypanosoma cruzi in San Pedro Department, Paraguay. Rev Bras Parasitol Vet. 2016;25:327-32. https://doi.org/10.1590/S1984-29612016052
Acosta N, López E, Lewis MD, Llewellyn MS, Gómez A, Román F, et al. Hosts and vectors of Trypanosoma cruzi discrete typing units in the Chagas disease endemic region of the Paraguayan Chaco. Parasitol. 2017;144:884-98. https://doi.org/10.1017/S0031182016002663
Camargo E. Growth and differentiation in Trypanosoma cruzi I. Origen of metacyclic trypanosomes in liquid media. Rev Inst Med Trop São Paulo. 1964;6:93-100.
Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, da Silva Valente SA, et al. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. Int J Parasitol. 2007;37:111-20. https://doi.org/10.1016/j.ijpara.2006.08.002
Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GA, et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225-33. https://doi.org/10.1016/j.ijpara.2004.10.024
Lewis MD, Ma J, Yeo M, Carrasco HJ, Llewellyn MS, Miles MA. Genotyping of Trypanosoma cruzi: Systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am J Trop Med Hyg. 2009;81:1041-9. https://doi.org/10.4269/ajtmh.2009.09-0305
Andrade SG, Magalhães JB, Pontes AL. Evaluation of chemotherapy with benznidazole and nifurtimox in mice infected with Trypanosoma cruzi strains of different types. Bull World Health Organ. 1985;63:721-6.
Muñoz-Calderón A, Santaniello A, Pereira A, Yannuzzi J, Díaz-Bello Z, Alarcón de Noya B. Susceptibilidad in vitro a nifurtimox y benznidazol de aislados de Trypanosoma cruzi obtenidos de pacientes venezolanos con enfermedad de Chagas infectados por mecanismos de transmisión oral y vectorial. Rev Ibero-Latinoam Parasitol. 2012;71:14-22.
Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci USA. 2008;105:5022-7. https://doi.org/10.1073/pnas.0711014105
Mejía-Jaramillo AM, Fernández GJ, Palacio L, Triana-Chávez O. Gene expression study using real-time PCR identifies an NTR gene as a major marker of resistance to benzonidazole in Trypanosoma cruzi. Parasit Vectors. 2011;4:169. https://doi.org/10.1186/1756-3305-4-169
Campos MC, Leon LL, Taylor MC, Kelly JM. Benznidazole-resistance in Trypanosoma cruzi: Evidence that distinct mechanisms can act in concert. Mol Biochem Parasitol. 2014;193:17-9. https://doi.org/10.1016/j.molbiopara.2014.01.002
González L, García-Huertas P, Triana-Chávez O, García GA, Murta SMF, Mejía-Jaramillo AM. Aldo-ketoreductase and alcohol dehydrogenase contribute to benznidazole natural resistance in Trypanosoma cruzi. Mol Microbiol. 2017;106:704-18. https://doi.org/10.1111/mmi.13830
García-Huertas P, Mejía-Jaramillo AM, Machado CR, Guimarães AC, Triana-Chávez O. Prostaglandin F2α synthase in Trypanosoma cruzi plays critical roles in oxidative stress and susceptibility to benznidazole. R Soc Open Sci. 2017a;4:170773. https://doi.org/10.1098/rsos.170773
Quebrada-Palacio LP, González MN, Hernandez-Vasquez Y, Perrone AE, Parodi-Talice A, Bua J, et al. Phenotypic diversity and drug susceptibility of Trypanosoma cruzi TcV clinical isolates. PLoS ONE. 2018;13:e020346. https://doi.org/10.1371/journal.pone.0203462
Campos MC, Castro-Pinto DB, Ribeiro GA, Berredo-Pinho MM, Gomes LH, da Silva Bellieny MS, et al. P-glycoprotein efflux pump plays an important role in Trypanosoma cruzi drug resistance. Parasitol Res. 2013;112:2341-51. https://doi.org/10.1007/s00436-013-3398-z
García-Huertas P, Mejía-Jaramillo AM, González L, Triana-Chávez O. Transcriptome and functional genomics reveal the participation of adenine phosphoribosyltransferase in Trypanosoma cruzi resistance to benznidazole. J Cell Biochem. 2017;118:1936-45. https://doi.org/10.1002/jcb.25978
Nozaki T, Engel JC, Dvorak JA. Cellular and molecular biological analyses of nifurtimox resistance in Trypanosoma cruzi. Am J Trop Med Hyg. 1996;55:111-7. https://doi.org/10.4269/ajtmh.1996.55.111
Toledo MJ, Bahia MT, Carneiro CM, Martins-Filho OA, Tibayrenc M, Barnabé C, et al. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob Agents Chemother. 2003;47:223-30. https://doi.org/10.1128/aac.47.1.223-230.2003
Villarreal D, Barnabè C, Sereno D, Tibayrenc M. Lack of correlation between in vitro susceptibility to benznidazole and phylogenetic diversity of Trypanosoma cruzi, the agent of Chagas disease. Exp Parasitol. 2004;108:24-31. https://doi.org/10.1016/j.exppara.2004.07.001
Teston AP, Monteiro WM, Reis D, BossolaniGleison DP, Gomes ML, de Araújo SM, et al. In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Trop Med Int Health. 2013;18:85-95. https://doi.org/10.1111/tmi.12014
Gruendling AP, Massago M, Teston AP, Monteiro WM, Kaneshima EN, Araújo SM, et al. Impact of benznidazole on infection course in mice experimentally infected with Trypanosoma cruzi I, II, and IV. Am J Trop Med Hyg. 2015;92:1178-89. https://doi.org/10.4269/ajtmh.13-0690
Wilkinson SR, Bot C, Kelly JM, Hall BS. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr Top Med Chem. 2011;11:2072-84. https://doi.org/10.2174/156802611796575894
Hall BS, Wilkinson SR. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother. 2012;56:115-23. https://doi.org/10.1128/AAC.05135-11
Moraes CB, Giardini MA, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, et al. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: Implications for Chagas disease drug discovery and development. Sci Rep. 2014;4:4703. https://doi.org/10.1038/srep04703
Cal M, Ioset JR, Fügi MA, Mäser P, Kaiser M. Assessing anti-T. cruzi candidates in vitro for sterile cidality. Int J Parasitol Drugs Drug Resist. 2016;6:165-70. https://doi.org/10.1016/j.ijpddr.2016.08.003
MacLean LM, Thomas J, Lewis MD, Cotillo I, Gray DW, De Rycker M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’disease drug discovery. PLoS Negl Trop Dis. 2018;12:e0006612. https://doi.org/10.1371/journal.pntd.0006612
Burgos LG, Ortiz BD, Canese A, Ojeda A, Melo M. Reactivation of Chagas disease y immunosuppressive therapy in a patient with systemic lupus erythematosus: Report of an exceptional case. Am J Dermatopathol. 2012;34:e84-9. https://doi.org/10.1097/DAD.0b013e318257f9e2
Vera de Bilbao N, Samudio M, Schinini A, Acosta N, López E, González N, et al. Evaluación a 24 meses post-tratamiento con benznidazol en niños de 6 a12 años infectados con Trypanosoma cruzi. Rev Patol Trop. 2004;33:301-12.
Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 2014;209:150-62. https://doi.org/10.1093/infdis/jit420
Diniz L de F, Urbina JA, de Andrade IM, Mazzeti AL, Martins TA, Caldas IS, et al. Benznidazole and posaconazole in experimental Chagas disease: Positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis. 2013;7:e2367. https://doi.org/10.1371/journal.pntd.0002367
Campos MC, Phelan J, Francisco AF, Taylor MC, Lewis MD, Pain A, et al. Genome-wide mutagenesis and multi-drug resistance in American trypanosomes induced by the front-line drug benznidazole. Sci Rep. 2017;7:14407. https://doi.org/10.1038/s41598-017-14986-6
Diniz LF, Caldas IS, Guedes PMM, Crepalde G, de Lana M, Carneiro CM, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agent Chemother. 2010;54:2979-86. https://doi.org/10.1128/AAC.01742-09
Caldas S, Caldas IS, Cecílio AB, Diniz LF, Talvani A, Ribeiro I, et al. Therapeutic responses to different anti-Trypanosoma cruzi drugs in experimental infection by benznidazole resistant stock. Parasitol. 2014;21:1-10. https://doi.org/10.1017/S0031182014000882
Some similar items:
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- María Clara Echeverry, Nubia Catalina Tovar, Guillermo Mora, Presence of antibodies to cardiac neuroreceptors in patients with Chagas disease , Biomedica: Vol. 29 No. 3 (2009)
- Dairo Alonso Rendón, Carlos M. Genes, Omar Triana, Myocardial cellular damage and the activity of the mitochondrial ATP synthase in rats infected with a Colombian strain of Trypanosoma cruzi , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Concepción Judith Puerta, Johana María Guevara, Paula Ximena Pavía, Marleny Montilla, Rubén Santiago Nicholls, Edgar Parra, Yuli Katherine Barrera, Evaluation of TcH2AF-R and S35-S36 primers in PCR tests for the detection of Trypanosoma cruzi in mouse cardiac tissue , Biomedica: Vol. 28 No. 4 (2008)
- Paula Ximena Pavía, Nubia Lucía Roa, Ana María Uribe, Concepción Judith Puerta, Using S35-S36 and TcH2AF-R primer-based PCR tests to follow-up a Chagas’ disease patient who had undergone a heart transplant , Biomedica: Vol. 31 No. 2 (2011)
- Richard Hoyos, Lisandro Pacheco, Luz Adriana Agudelo, German Zafra, Pedro Blanco, Omar Triana, Seroprevalence of Chagas disease and associated risk factors in a population of Morroa, Sucre , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Concepción Judith Puerta, Paula Ximena Pavia, Marleny Montilla, Carolina Flórez, Giomar Herrera, Juan Manuel Ospina, Fred Manrique, Rubén Santiago Nicholls, The first case of congenital Chagas’ disease analyzed by AP-PCR in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Luz Adriana Botero, Ana María Mejía, Omar Triana, Biological and genetic characterization of two Colombian clones of Trypanosoma cruzi groups I and II , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Sandra Paola Santander, Adriana Cuéllar, María del Carmen Thomas, Fanny Guzmán, Alberto Gómez, Manuel Carlos López, Concepción Puerta, Expression of markers on dendritic cells from chronic chagasic patients stimulated with the KMP-11 protein and the K1 peptide from Trypanosoma cruzi , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Rubén Santiago Nicholls, Zulma Milena Cucunubá, Angélica Knudson, Astrid Carolina Flórez, Marleny Montilla, Concepción Judith Puerta, Paula Ximena Pavía, Acute Chagas disease in Colombia: a rarely suspected disease. Report of 10 cases presented during the 2002-2005 period , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























