Genetic variability of Aedes aegypti in the department of Sucre, Colombia, by analysis of the nucleotide sequence of the mitochondrial ND4 gene
Abstract
Introduction. Aedes aegypti is the most important mosquito species in America for the transmission of viruses of dengue, Zika, Chikungunya and yellow fever. Ecological factors as well as chemical controls can affect the genetic composition of Ae. aegypti populations, which is why its genetic characterization is necessary.
Objective. To determine the genetic variability of Ae. aegypti populations in four municipalities of Sucre department, Colombia.
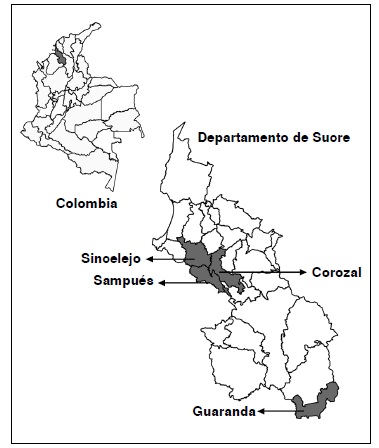
Materials and methods. Larvae of Ae. aegypti, collected in the municipalities of Sincelejo, Sampués, Corozal and Guaranda, Sucre department, were reared under laboratory conditions to adult stage. A segment of the mitochondrial ND4 gene which codes for the subunit 4 of the enzyme NADH-dehydrogenase was used as genetic marker. The genetic analysis included the estimation of parameters of nucleotide and haplotype diversity, genetic structure and gene flow.
Results. One hundred and eight partial sequences of 357 nucleotides and four nucleotide haplotypes of the ND4 gene of Ae. aegypti were obtained. A significantly high genetic differentiation was found between the Sampués and Guaranda populations (FST=0.59467), Sincelejo and Sampués (FST=0.25637), and Corozal and Guaranda (FST=0.22237). A high gene flow (Nm=infinite) was observed among the populations of Sincelejo and Corozal.
Conclusion. There are genetic differences between the Ae. aegypti populations from the municipalities of Sucre department. The presence of a new haplotype of the mitochondrial ND4 gene of Ae. aegypti in Colombia was recorded, detected in the municipality of Sincelejo.
Downloads
References
Organización Mundial de la Salud. Dengue: guías para diagnóstico, tratamiento, prevención y control. OMS; 2009. Fecha de consulta: 12 de septiembre de 2015. Disponible en: http://apps.who.int/iris/bitstream/10665/44504/1/9789995479213_spa.pdf?ua=1
Aitken TH, Downs WG, Shope RE. Aedes aegypti strain fitness for yellow fever virus transmission. Am J Trop Med Hyg. 1977;26:985-9. https://doi.org/10.4269/ajtmh.1977.26.985
Bennett KE, Olson KE, Muñoz M, Fernández-Salas I, Farfán-Ale JA, Higgs S, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg. 2002;67:85-92. https://doi.org/10.4269/ajtmh.2002.67.85
World Health Organization. Guidelines for prevention and control of chikungunya fever. World Health Organization. Fecha de consulta: 13 de mayo de 2016. Disponible en: http://www.wpro.who.int/mvp/topics/ntd/Chikungunya_WHO_SEARO.pdf.
Bosio CF, Beaty BJ, Black WC. Quantitative genetics of vector competence for dengue-2 virus in Aedes aegypti. Am J Trop Med Hyg. 1998;59:965-70. https://doi.org/10.4269/ajtmh.1998.59.965
Anderson JR, Rico-Hesse R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg. 2006;75:886-92. https://doi.org/10.4269/ajtmh.2006.75.886
Quintero D, Osorio J, Martínez M. Competencia vectorial: consideraciones entomológicas y su influencia sobre la epidemiología del Dengue. Iatreia. 2010;23:146-56.
Lozano S, Fernández I, Muñoz M, García J, Olson K, Beaty B, et al. The neovolcanic axis is a barrier to gene flow among Aedes aegypti populations in mexico that differ in vector competence for dengue 2 virus. PLoS Negl Trop Dis. 2009;3:e468. https://doi.org/10.1371/journal.pntd.0000468
Ravela S, Montenyb N, Velasco D, Verdugob J, Cunya G. A preliminary study of the population genetics of Aedes aegypti (Diptera: Culicidae) from Mexico using microsatellite and AFLP markers. Acta Trop. 2001;78:241-50. https://doi.org/10.1016/S0001-706X(01)00083-3
Yan G, Chadee D, Severson D. Evidence for genetic hitchhiking effect associated with insecticide resistance in Aedes aegypti. Genetics. 1998;148:793-800.
Gorrochotegui-Escalante N, Gómez-Machorro C, Lozano-Fuentes S, Fernández-Salas L, Muñoz M, Farfán-Ale JA, et al. Breeding structure of Aedes aegypti populations in Mexico varies by region. Am J Trop Med Hyg. 2002;66:213-22. https://doi.org/10.4269/ajtmh.2002.66.213
Ocampo C, Wesson D. Population dynamics of Aedes aegypti from a dengue hyperendemic urban setting in Colombia. Am J Trop Med Hyg. 2004;71:506-13. https://doi.org/10.4269/ajtmh.2004.71.506
Julio NB, Chiappero MB, Rossi HJ, Rondan-Dueñas JC, Gardenal CN. Genetic structure of Aedes aegypti in the city of Córdoba (Argentina), a recently reinfested area. Mem Inst Oswaldo Cruz. 2009;104:626-31. http://dx.doi.org/10.1590/S0074-02762009000400016
Sylla M, Bosio C, Urdaneta-Márquez L, Ndiaye M, Black WC. Gene flow, subspecies composition, and dengue virus-2 susceptibility among Aedes aegypti collections in Senegal. PLoS Negl Trop Dis. 2009;3:e408. https://doi.org/10.1371/journal.pntd.0000408
Da Costa-Ribeiro M, Lourenço-de-Oliveira R, Failloux A. Low gene flow of Aedes aegypti between dengue-endemic and dengue-free areas in Southeastern and Southern Brazil. Am J Trop Med Hyg. 2007;77:303-9. https://doi.org/10.4269/ajtmh.2007.77.303
Leiva N, Cáceres O. Variabilidad genética de Aedes aegypti en algunas áreas del Perú usando Single Stranded Conformational Polymorphism (SSCP). Rev Peru Med Exp Salud Pública. 2004;21:158-66.
Da Costa-Ribeiro M, Lourenço-de-Oliveira R, Failloux A. Higher genetic variation estimated by microsatellites compared to isoenzyme markers in Aedes aegypti from Rio de Janeiro. Mem Inst Oswaldo Cruz. 2006;101:917-21. http://dx.doi.org/10.1590/S0074-02762006000800015.
Paupy C, Le Goff G, Brengues C, Guerra M, Revollo J, Barja Z, et al. Genetic structure and phylogeography of Aedes aegypti, the dengue and yellow-fever mosquito vector in Bolivia. Infect Genet Evol. 2012;12(6):1260–9. https://doi.org/10.1016/j.meegid.2012.04.012
Olanratmanee P, Kittayapong P, Chansang C, Hoffmann AA, Weeks AR, Endersby NM. Population genetic structure of Aedes (Stegomyia) aegypti (L.) at a micro-spatial scale in Thailand: Implications for a dengue suppression strategy. PLoS Negl Trop Dis. 2013;7:e1913. https://doi.org/10.1371/journal.pntd.0001913
Costa-da-Silva A, Capurro M, Bracco JE. Genetic lineages in the yellow fever mosquito Aedes (Stegomyia) aegypti (Diptera: Culicidae) from Peru. Mem Inst Oswaldo Cruz. 2005:100:639-44. http://dx.doi.org/10.1590/S0074-02762005000600007
Bosio CF, Harrington LC, Jones JW, Sithiprasasna R, Norris DE, Scott TW. Genetic structure of Aedes aegypti populations in Thailand using Mitochondrial DNA. Am J Trop Med Hyg. 2005;72:434-42. https://doi.org/10.4269/ajtmh.2005.72.434
Bracco J, Capurro M, Lourenço-de-Oliveira R, Mureb-Sallum M. Genetic variability of Aedes aegypti in the Américas using o mitochondrial gene: Evidence of multiple introductions. Mem Inst Oswaldo Cruz. 2007;102:573-80. http://dx.doi.org/10.1590/S0074-02762007005000062
Paduan K, Ribolla P. Mitochondrial DNA polymorphism and heteroplasmy in populations of Aedes aegypti in Brazil. J Med Entomol. 2008;45:59-67. https://doi.org/10.1093/jmedent/45.1.59
Lima R, Scarpassa V. Evidence of two lineages of the dengue vector Aedes aegypti in the Brazilian Amazon, based on mitochondrial DNA ND4 gene sequences. Genet Mol Biol. 2009;32:414-22. http://dx.doi.org/10.1590/S1415-47572009005000036
Caldera S, Jaramillo MC, Cochero S, Pérez-Doria A, Bejarano EE. Diferencias genéticas entre poblaciones de Aedes aegypti de municipios del Norte de Colombia, con baja y alta incidencia de dengue. Biomédica. 2013;33:89-98. http://dx.doi.org/10.7705/biomedica.v33i0.1573
Albrieu G, Gardenal N. Phylogeography of Aedes aegypti in Argentina: long-distance colonization and rapid restoration of fragmented relicts after a continental control campaign. Vector Borne Zoonotic Dis. 2012;12:254-61. https://doi.org/10.1089/vbz.2011.0696
Damal K, Murrell E, Juliano S, Conn J, Loew S. Phylogeography of Aedes aegypti (yellow fever mosquito) in South Mlorida: mtDNA evidence for human-aided dispersal. Am J Trop Med Hyg. 2013;89:482-88. https://doi.org/10.4269/ajtmh.13-0102
Scarpassa V, Bacry T, Cardoso R. Population genetics and phylogeography of Aedes aegypti (Diptera: Culicidae) from Brazil. Am J Trop Med Hyg. 2008;78:895–903. https://doi.org/10.4269/ajtmh.2008.78.895
Cadavid J, Rúa G, Campo O, Bedoya G, Rojas W. Cambios genéticos temporales y microgeográficos de Aedes aegypti en Medellín, Colombia. Biomédica. 2015;35:53-6. https://doi.org/10.7705/biomedica.v35i1.2343
Hoyos-López R, Pardo SR, Castaño JC, Gallego-Gómez JC. Código de barras para la tipificación de culícidos inmaduros de Armenia y Circasia (Quindío, Colombia). Rev Colomb Entomol. 2015;41:218-27. ISSN 0120-0488.
Rueda L. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue virus transmission. Zootaxa. 2004;589:1-60. http://dx.doi.org/10.11646/zootaxa.589.1.1
Atencia M, Pérez M, Jaramillo M, Caldera S, Bejarano E. Primer reporte de la mutación F1534C asociada con resistencia cruzada a DDT y piretroides en Aedes aegypti en Colombia. Biomédica. 2016;36:432-7. http://dx.doi.org/10.7705/biomedica.v36i3.2834
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731-739. https://doi.org/10.1093/molbev/msr121
Altschul S, Gish W, Miller W, Myers E, Lipman D. Blast BLAST. Basic local alignment search tool. J Mol Biol 1990; 215:403-10. https://doi.org/10.1016/S0022-2836(05)80360-2
Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451-2. https://doi.org/10.1093/bioinformatics/btp187
Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics.1983;105:437-60.
Fu Y, Li W. Statistical tests of neutrality mutations. Genetics. 1993;133:693-709.
Fluxus Technology Ltd. NETWORK 4.6.1.1. Steiner (MP) algorithm developed by Tobias Polzin and Siavash Vahdati Daneshmand. Fecha de consulta: 20 de noviembre de
Disponible en: http://www.fluxusengineering.com
Bandelt H, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37-48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Excoffier L, Lischer H. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564-67.
Mantel N. The detection of disease clustering and a geneized regression approach. Cancer Res. 1967;27:209-20.
Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219-28.
Cavalcanti M. MANTEL v1.19. Centro de Ciências Biológicas, Universidade Santa Úrsula. 2008. Fecha de consulta: 15 de septiembre de 2017. Disponible en: http://life.bio.sunysb.edu/morph/morphmet/mantel32.exe
Jaimes J, Arboleda S, Triana O, Gómez A. Spatiotemporal distribution of Aedes aegypti (Diptera: Culicidae) mitochondrial lineages in cities with distinct dengue incidence rates suggests complex population dynamics of the dengue vector in Colombia. PLoS Negl Trop Dis. 2015;9:e0003553. https://doi.org/10.1371/journal.pntd.0003553
Ministerio de Salud y Protección Social, República de Colombia. Situación actual de Dengue a semana 12 de 2013 periodo de análisis: 2008-2013. Fecha de consulta: 15 de septiembre de 2017. Disponible en: https://www.minsalud.gov.co/Documentos%20y%20Publicaciones/INFORME%20SITUACION%20DE%20DENGUE.pdf
Twerdochlib A, Dalla A, Leite S, Chitolina R, Westphal B, Navarro M. Genetic variability of a population of Aedes aegypti from Paraná, Brazil, using the mitochondrial ND4 gene. Rev Bras Entomol. 2012;56:249-56. http://dx.doi.org/10.1590/S0085-56262012005000030
Brito R, Manfrin M, Sene F. Mitochondrial DNA phylogeography of Brazilian populations of Drosophila buzzatii. Genet Mol Biol. 2002;25:161-71. http://dx.doi.org/10.1590/S1415-47572002000200009
Iturbe U. Adaptaciones y adaptación biológica. Sesbe. 2010;5:5-12.
Wright S. Evolution and the genetics of population, variability within and among natural populations. Chicago: The University of Chicago Press; 1978. p. 4.
Wright S. The genetical structure of populations. Chicago: University of Chicago Press. Annals of Eugenics. 1951;15:323-54.
Nelson MJ. Aedes aegypti: Biología y ecología. Washington: Organización Panamericana de la Salud; 1986. p. 1-50
Some similar items:
- Tania Camacho, Fernando de la Hoz, Victor Cárdenas, Carmen Sánchez, Laura de Calderón, Ligia Pérez, Antonio Bermúdez, Incomplete surveillance of a dengue-2 epidemic in Ibagué, Colombia, 1995-1997. , Biomedica: Vol. 24 No. 2 (2004)
- Laura Cabezas, Wilson Cabanzo, Fernando Santa, Victor Alberto Olano, Diana Sarmiento, Sandra Vargas, Juan Felipe Jaramillo, Thor-Axel Stenstrom, Hans J. Overgaard, María Inés Matiz, Spatial distribution of Aedes aegypti (Diptera: Culicidae) in the rural area of two municipalities of Cundinamarca, Colombia , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Marcela Conde, Lorena I. Orjuela, Cesar Augusto Castellanos, Manuela Herrera-Varela, Susana Licastro, Martha L. Quiñones, Insecticide susceptibility evaluation in Aedes aegypti populations of Caldas, Colombia, in 2007 and 2011 , Biomedica: Vol. 35 No. 1 (2015)
- Érika Patricia Alarcón, Ángela María Segura, Guillermo Rúa-Uribe, Gabriel Parra-Henao, Ovitraps evaluation for surveillance and control of Aedes aegypti in two urban settlements of Urabá, Antioquia , Biomedica: Vol. 34 No. 3 (2014)
- José Joaquín Carvajal, Nildimar Alves Honorio, Silvia Patricia Díaz, Edinso Rafael Ruiz, Jimmy Asprilla, Susanne Ardila, Gabriel Parra-Henao, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the municipality of Istmina, Chocó, Colombia , Biomedica: Vol. 36 No. 3 (2016)
- Sandy Milena Caldera, María Cristina Jaramillo, Suljey Cochero, Alveiro Pérez-Doria, Eduar Elías Bejarano, Genetic differences between populations of Aedes aegypti from municipalities in northern Colombia, with high and low dengue incidence , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Freddy Ruiz-López, Ana González-Mazo, Andrés Vélez-Mira, Giovan F. Gómez, Luisa Zuleta, Sandra Uribe, Iván Darío Vélez-Bernal, Presence of Aedes (Stegomyia) aegypti (Linnaeus, 1762) and its natural infection with dengue virus at unrecorded heights in Colombia , Biomedica: Vol. 36 No. 2 (2016)
- Berta Nelly Restrepo, Leidy Diana Piedrahita, Ivony Yireth Agudelo, Katherine Marín, Ruth Ramírez, Dengue infection: A common cause of febrile syndrome in patients from Quibdó, Chocó, Colombia , Biomedica: Vol. 35 No. 1 (2015)
- Berta Nelly Restrepo, Margarita Arboleda, Ruth Ramírez, Gonzalo Álvarez, Serum platelet-activating factor acetylhydrolase activity in dengue patients of African or mestizo descendency , Biomedica: Vol. 31 No. 4 (2011)
- María Elena Cuéllar-Jiménez, Olga Lucía Velásquez-Escobar, Ranulfo González-Obando, Carlos Andrés Morales-Reichmann, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia , Biomedica: Vol. 27 No. 2 (2007)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |