The porcine biomodel in translational medical research: From biomodel to human lung transplantation
Abstract
Introduction: Human and porcine anatomy are comparable. In consequence, the porcine biomodel has the potential to be implemented in the training of surgical professionals in areas such as solid organ transplantation.
Objectives: We described the procedures and findings obtained in the experiments of translational respiratory medicine with the porcine biomodel, within an experimentation animal laboratory, and we present a comparative review between human and porcine lung.
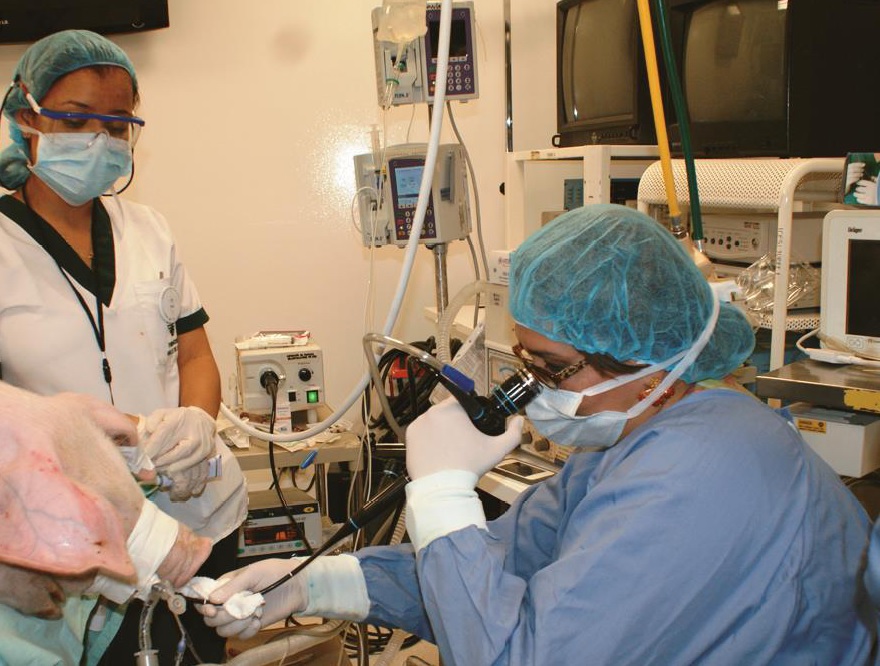
Materials and methods: The experiment was done in nine pigs of hybrid race within a laboratory of experimental surgery. The anatomy and histology of the respiratory tract were studied with fibrobronchoscopy, bronchial biopsy and bronchoalveolar lavage. The bronchoalveolar lavage was studied with liquid-based cytology and assessed with Papanicolau and hematoxylin-eosin staining. Molecular pathology techniques such as immunohistochemistry, flow cytometry, and electronic microscopy were implemented. The pigs were subjected to left pneumonectomy with posterior implantation of the graft into another experimental pig.
Results: Histopathologic and molecular studies evidenced predominance of alveolar macrophages (98%) and T-lymphocytes (2%) in the porcine bronchoalveolar lavage. Studies on the porcine lung parenchyma revealed hyperplasic lymphoid tissue associated with the bronchial walls. Electronic microscopy evidenced the presence of T-lymphocytes within the epithelium and the cilia diameter was similar to the human.
Conclusions: The porcine biomodel is a viable tool in translational research applied to the understanding of the respiratory system anatomy and the training in lung transplantation. The implementation of this experimental model has the potential to strength the groups who plan to implement an institutional program of lung transplantation in humans.
Downloads
References
Walters EM, Wolf E, Whyte JJ, Mao J, Renner S, Nagashima H, et al. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Med Genomics. 2012;5:55. https://doi.org/10.1186/1755-8794-5-55
Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240-63. https://doi.org/10.1152/ajplung.90203.2008
Bruun CS, Jensen LK, Leifsson PS, Nielsen J, Cirera S, Jørgensen CB, et al. Functional characterization of a porcine emphysema model. Lung. 2013;191:669-75. https://doi.org/10.1007/s00408-013-9504-2
Borges JB, Costa EL, Suárez-Sipmann F, Widström C, Larsson A, Amato M, et al. Early inflammation mainly affects normally and poorly aerated lung in experimental ventilatorinduced lung injury. Crit Care Med. 2014;42:e279-87. https://doi.org/10.1097/CCM.0000000000000161
Kita YF, Ando A, Tanaka K, Suzuki S, Ozaki Y, Uenishi H, et al. Application of highresolution, massively parallel pyrosequencing for estimation of haplotypes and gene expression levels of swine leukocyte antigen (SLA) class I genes. Immunogenetics. 2012;64:187-99. https://doi.org/10.1007/s00251-011-0572-2
Snell GI, Paraskeva M, Westall GP. Donor selection and management. Semin Respir Crit Care Med. 2013;34:361-70. https://doi.org/10.1055/s-0033-1348464
Karimi A, Cobb JA, Staples ED, Baz MA, Beaver TM. Technical pearls for swine lung transplantation. J Surg Res. 2011;171:e107-11. https://doi.org/10.1016/j.jss.2011.05.067
Collins AM, Rylance J, Wootton DG, Wright AD, Wright AK, Fullerton DG, et al. Bronchoalveolar lavage (BAL) for research; obtaining adequate sample yield. J Vis Exp. 2014. https://doi.org/10.3791/4345
Bufalari A, De Monte V, Pecoriello R, Donati L, Ceccarelli S, Cagini L, et al. Experimental left pneumonectomy in pigs: Procedure and management. J Surg Res. 2015;198:208-16. https://doi.org/10.1016/j.jss.2015.05.045
Lama VN, Belperio JA, Christie JD, El-Chemaly S, Fishbein MC, Gelman AE, et al. Models of lung transplant research: A consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight. 2017;2:1-14. https://doi.org/10.1172/jci.insight.93121
Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:1-9. https://doi.org/10.1126/scitranslmed.3000266
Daggett CW, Yeatman M, Lodge AJ, Chen EP, Linn SS, Gullotto C, et al. Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg. 1998;115:19-27. https://doi.org/https://doi.org/10.1016/S0022-5223(98)70438-6
Hartmann JF, Hutchison CF, Jewell ME. Pig bronchial mucous membrane: A model system for assessing respiratory mucus release in vitro. Exp Lung Res. 1984;6:59-70.
Hartert M, Senbaklavacin O, Gohrbandt B, Fischer BM, Buhl R, Vahld CF. Lung transplantation: A treatment option in end-stage lung disease. Dtsch Arztebl Int. 2014;111:107-16. https://doi.org/10.3238/arztebl.2014.0107
Haworth SG, Hislop AA. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res. 1981;15:108-19.
Davies G, Reid L. Growth of the alveoli and pulmonary arteries in childhood. Thorax. 1970;25:669-81. https://doi.org/10.1136/thx.25.6.669
Florens M, Sapoval B, Filoche M. An anatomical and functional model of the human tracheobronchial tree. J Appl Physiol (1985). 2011;110:756-63. https://doi.org/10.1152/japplphysiol.00984.2010
Maina JN, van Gils P. Morphometric characterization of the airway and vascular systems of the lung of the domestic pig, Sus scrofa: comparison of the airway, arterial and venous systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:781-98. https://doi.org/10.1016/S1095-6433(01)00411-1
Cohen BS, Sussman RG, Lippmann M. Factors affecting distribution of airflow in a human tracheobronchial cast. Respir Physiol. 1993;93:261-78.
Noble PB, McLaughlin RA, West AR, Becker S, Armstrong JJ, McFawn PK, et al. Distribution of airway narrowing responses across generations and at branching points, assessed in vitro by anatomical optical coherence tomography. Respir Res. 2010;11:9. https://doi.org/10.1186/1465-9921-11-9
Menkes HA, Macklem PT. Collateral Flow. Suppl 12. Handbook of Physiology, The Respiratory System, Mechanics of Breathing. 2011. Wiley Online Library. p. 337-53. https://doi.org/10.1002/cphy.cp030321
Peake JL, Pinkerton KE. Gross and subgross anatomy of lungs, pleura, connective tissue septa, distal airways, and structural units A2. In: Parent RA, Elsevier Inc. Comparative biology of the normal lung. Second edition. San Diego: Academic Press; 2015. p. 21-31. https://doi.org/10.1016/B978-0-12-404577-4.00003-5
Woolcock AJ, Macklem PT. Mechanical factors influencing collateral ventilation in human, dog, and pig lungs. J Appl Physiol. 1971;30:99-115. https://doi.org/10.1152/jappl.1971.30.1.99
Nakakuki S. Bronchial tree, lobular division and blood vessels of the pig lung. J Vet Med Sci. 1994;56:685-9.
Siegel MJ, Shackelford GD, Francis RS, McAlister WH. Tracheal bronchus. Radiology. 1979;130:353-5. https://doi.org/10.1148/130.2.353
Dondelinger RF, Ghysels MP, Brisbois D, Donkers E, Snaps FR, Saunders J, et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur Radiol. 1998;8:1254-73. https://doi.org/10.1007/s003300050545
Schummer A, Nickel R, Sack WO. The viscera of the domestic mammals. Berlín: Springer-Verlag; 1979. p. 211- 279.
Amis TC, McKiernan BC. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Res. 1986;47:2649-57.
Boyden EA. Segmental anatomy of the lungs: A study of the patterns of the segmental bronchi and related pulmonary vessels. New York and London: McGraw-Hill; 1955. p. 108.
Judge EP, Hughes JM, Egan JJ, Maguire M, Molloy EL, O'Dea S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol. 2014;51:334-43. https://doi.org/10.1165/rcmb.2013-0453TR
Jones TC, Hunt RD, King NW. Veterinary pathology. Baltimore: Lippincott Williams & Wilkins; 1997. 1392 p.
Baskerville A. Histological and ultrastructural observations on the development of the lung of the fetal pig. Acta Anat (Basel). 1976;95:218-33.
Crews A, Taylor AE, Ballard ST. Liquid transport properties of porcine tracheal epithelium. J Appl Physiol. 2001;91:797-802.
Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol. 2007;36:313-23. https://doi.org/10.1165/rcmb.2006-0286OC
Some similar items:
- Vanihamín Domínguez, Itzen Aguiñiga, Leticia Moreno, Beatriz Torres, Edelmiro Santiago-Osorio, Sodium caseinate increases the number of B lymphocytes in mouse , Biomedica: Vol. 37 No. 4 (2017)
- Juan Carlos Villa-Camacho, Juan Camilo Vargas-Zambrano, John Mario González, Flow cytometry model for the detection of neutralizing antibodies against of IFN-β , Biomedica: Vol. 32 No. 4 (2012)
- Viviana Marcela Rodríguez, Adriana Cuéllar, Lyda Marcela Cuspoca, Carmen Lucía Contreras, Marcela Mercado, Alberto Gómez, Phenotypical determinants of stem cell subpopulations derived from human umbilical cord blood. , Biomedica: Vol. 26 No. 1 (2006)
- Lina Marcela Barrera, León Darío Ortiz, Hugo Grisales, Mauricio Rojas, Mauricio Camargo, Flow cytometry in peripheral blood reticulocytes as a marker of chromosome instability in highgrade glioma patients , Biomedica: Vol. 38 No. 3 (2018)
- Lina Echeverri-Toro, Andrés Arango, Sigifredo Ospina, Carlos Agudelo, Bordetella bronchiseptica recurrent bacteraemia in a patient with bone marrow transplantation , Biomedica: Vol. 35 No. 3 (2015)
- Ana María Arrunátegui, Daniel S. Ramón, Luz Marina Viola, Linda G. Olsen, Andrés Jaramillo, Technical and clinical aspects of the histocompatibility crossmatch assay in solid organ transplantation , Biomedica: Vol. 42 No. 2 (2022)
- Mike Celis, Yohanna Navarro, Norma Serrano, Daniel Martínez, Wendy Nieto , B-cell lymphocytosis in relatives of Colombian patients with chronic B-cell lymphoproliferative disorders , Biomedica: Vol. 43 No. Sp. 3 (2023): Enfermedades crónicas no transmisibles
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























