Molecular detection of pathogenic Leptospira in synanthropic and wild rodents captured in Yucatán, México
Abstract
Introduction: Leptospirosis is a zoonotic disease caused by bacteria of the genus Leptospira, which is endemic in México and considered a public and veterinary health problem. Rodents are the most relevant reservoirs of Leptospira spp. because the bacteria establish and reproduce in its renal tissue and are excreted through the urine.
Objective: To identify the presence of Leptospira spp. in renal tissue from rodents captured in Yucatán, México.
Materials and methods: Synanthropic and wild rodents were captured in the rural municipality of Cenotillo, Yucatán, México. We collected one kidney from each rodent and extracted the total DNA. The identification of Leptospira spp. was done by detecting two fragments of the 16S rRNA gene using end-point polymerase chain reaction (PCR). We sequenced and analyzed positive products using alignment tools.
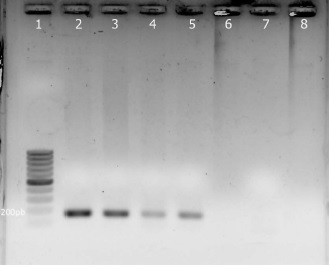
Results: A total of 92 rodents belonging to seven different species were captured. The PCR yielded a global positivity of 5.4% (5/92). The alignment analysis of the sequenced products demonstrated a 100% of coverage and identity with Leptospira interrogans. This is the first molecular evidence of Leptospira spp. circulation in Heteromys gaumeri captured in Yucatán, México.
Conclusion: Our results evidenced that rodents of Yucatán are reservoirs of Leptospira spp. and participate in the infection cycle of leptospirosis in the region.
Downloads
References
Ko AI, Goarant C, Picardeau M. Leptospira: The dawn of the molecular genetics era for emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736-47. https://doi.org/10.1038/nrmicro2208
Yalin W, Lingbing Z, Hongliang Y, Jianmin X, Xiangyan Z, Xiaokui G, et al. High prevalence of pathogenic Leptospira in wild and domesticated animals in an endemic area of China. Asian Pac J Trop Med. 2011;4:841-5. https://doi.org/10.1016/S1995-7645(11)60205-8
Torres-Castro M, Hernández-Betancourt S, Agudelo-Flórez P, Arroyave-Sierra E, Zavala-Castro J, Puerto IF. Revisión actual de la epidemiología de la leptospirosis. Rev Med Inst Mex Seguro Soc. 2016;54:620-5.
Sánchez-Montes S, Espinosa-Martínez DV, Ríos-Muñoz CA, Berzunza-Cruz M, Becker I. Leptospirosis in Mexico: Epidemiology and potential distribution of human cases. PLoS One. 2015;10:e0133720. https://doi.org/10.1371/journal.pone.0133720
Torres-Castro MA, Gutiérrez-Ruiz E, Hernández-Betancourt S, Peláez-Sánchez R, Agudelo-Flórez P, Guillermo-Cordero L, et al. First molecular evidence of Leptospira spp. in synanthropic rodents captured in Yucatán, México. Revue Méd Vét. 2014;165:213-8.
Torres-Castro M, Guillermo-Cordero L, Hernández-Betancourt S, Gutiérrez-Ruiz E, Agudelo-Flórez P, Peláez-Sánchez R, et al. First histopathological study in kidneys of rodents naturally infected whit Leptospira pathogenic species from Yucatán, México. Asian Pac J Trop Med. 2016;9:145-7. https://doi.org/10.1016/j.apjtm.2016.01.018
Ruiz-Piña HA, Puc-Franco MA, Flores-Abuxapqui J, Vado-Solis I, Cárdenas-Marrufo MF. Isolation of Salmonella enterica and serologic reactivity to Leptospira interrogans in opossums (Didelphis virginiana) from Yucatán, México. Rev Inst Med trop Sao Paulo. 2002;44:235-7. https://doi.org/10.1590/S0036-46652002000400011
Cárdenas-Marrufo MF, Vado-Solís I, Pérez-Osorio CE, Segura-Correa JC. Seropositivity to leptospirosis in domestic reservoirs and detection of Leptospira spp. from water sources, in farms of Yucatán, México. Trop Subtrop Agroecosyst. 2011;14:185-9.
Jiménez-Coello M, Vado-Solís I, Cárdenas-Marrufo MF, Rodríguez-Buenfil JC, Ortega-Pacheco A. Serological survey of canine leptospirosis in the tropics of Yucatán, México, using two different tests. Acta Trop. 2008;106:22-6. https://doi.org/10.1016/j.actatropica.2007.12.011
Enciclopedia de los Municipios y Delegaciones de México. Estado de Yucatán. Fecha de consulta: 10 de enero de 2017. Disponible en: http://www.inafed.gob.mx/work/enciclopedia/EMM31yucatan/municipios/31012a.html
Sikes RS, Gannon WL, Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92:235-53. https://doi.org/10.1644/10-MAMM-F-355.1
Norma Oficial Mexicana NOM.062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación. México. Fecha de consulta: 10 de enero de 2017. Disponible en: http://www.ibt.unam.mx/computo/pdfs/bioterio.NOM-062.pdf
Leary S, Underwood W, Lilly E, Anthony R, Cartner S, Corey S, et al. AVMA guidelines for the euthanasia of animals. Schaumburg, Illinois: American Veterinary Medical Association; 2013. p. 18-41.
Haake DA, Suchard MA, Kelley MM, Dundoo M, Alt DP, Zuerner RL. Molecular evolution and mosaicism of leptospiral outer membrane proteins involves horizontal DNA transfer. J Bacteriol. 2004;186:2818-28. https://doi.org/10.1128/JB.186.9.2818-2828.2004
Shukla J. 16S rRNA PCR for differentiation of pathogenic and non-pathogenic leptospira isolates. Indian J Med Microbiol. 2003;21:25-30.
Backstedt BT, Buyuktanir O, Lindow J, Wunder EA Jr, Reis MG, Usmani-Brown S, et al. Efficient detection of pathogenic leptospires using 16S ribosomal RNA. PLoS One. 2015;10:e0128913. https://doi.org/10.1371/journal.pone.0128913
Torres-Castro M. Estudio sobre roedores sinantrópicos como reservorios de patógenos zoonóticos en Yucatán. Rev Biomed. 2017;28:89-115.
Panti-May A, Hernández-Betancourt S, Ruiz-Piña H, Medina-Peralta S. Abundance and population parameters of commensal rodents present in rural households in Yucatán, México. Int Biodeter Biodegr. 2012;66:77-81. https://doi.org/10.1016/j.ibiod.2011.10.006
Panti-May JA, Hernández-Betancourt SF, Torres-Castro MA, Machain-Williams C, Cigarroa-Toledo N, Sodá L, et al. Population characteristics of human-commensal rodents present in households from Mérida, Yucatán, México. Manter (Linc). 2016. Fecha de consulta: 10 de enero de 2017. Disponible en: https://digitalcommons.unl.edu/parasitology-manterlab/
Zavala-Velázquez JE, Vado-Solís IA, Rodríguez-Félix ME, Rodríguez-Angulo EM, Barrera-Pérez MA, Guzmán-Marín ES. Leptospirosis anictérica en un brote epidémico de dengue en la península de Yucatán. Rev Biomed. 1998;9:78-83.
Vado-Solís I, Cárdenas-Marrufo MF, Jiménez-Delgadillo B, Alzina-López A, Laviada-Molina H, Suárez-Solís V, et al. Clinical-epidemiological study of leptospirosis in humans and reservoirs in Yucatán, México. Rev Inst Med Trop Sao Paulo. 2002;44:335-40. https://doi.org/10.1590/S0036-46652002000600008
Zavala-Velázquez J, Cárdenas-Marrufo M, Vado-Solís I, Cetina-Cámara M, Cano-Tur J, Laviada-Molina H. Hemorrhagic pulmonary leptospirosis: Three cases from the Yucatán peninsula, México. Rev Soc Bras Med Trop. 2008;41:404-8. https://doi.org/10.1590/S0037-86822008000400016
Ortega-Pacheco A, Colin-Flores RF, Gutiérrez-Blanco E, Jiménez-Coello M. Frequency and type of renal lesions in dogs naturally infected with Leptospira species. Ann N Y Acad Sci. 2008;1149:270-274. https://doi.org/10.1196/annals.1428.088
Reyes-Novelo E, Ruiz-Piña H, Escobedo-Ortegón J, Rodríguez-Vivas I, Bolio-González M, Polanco-Rodríguez Á, et al. Situación actual y perspectivas para el estudio de las enfermedades zoonóticas emergentes y reemergentes y olvidadas de la península de Yucatán, México. Trop Subtrop Agroecosys. 2011;14:35-54.
Lilenbaum W, Monteiro RV, Albuquerque CE, Ristow P, Fraguas S, Cardoso VS, et al. Leptospiral antibodies in wild felines from Rio de Janeiro Zoo, Brazil. Vet J. 2004;168:191-3. https://doi.org/10.1016/S1090-0233(03)00139-4
Vieira AS, Narduche L, Martins G, Schabib-Péres IAHF, Zimmermann NP, Juliano RS, et al. Detection of wild animals as carriers of Leptospira by PCR in the Panantal biome, Brazil. Acta Trop. 2016;163:87-9. https://doi.org/10.1016/j.actatropica.2016.08.001
Panti-May JA, Torres-Castro M, Hernández-Betancourt S, Dzul-Rosado K, Zavala-Castro J, López-Ávila K, et al. Detection of Rickettsia felis in wild mammals from three municipalities in Yucatán, México. Ecohealth. 2015;12:523-7. https://doi.org/10.1007/s10393-014-1003-2
Espinosa-Martínez DV, Sánchez-Montes DS, León-Paniagua L, Ríos-Muñoz CA, Berzunza-Cruz M, Becker I. New wildlife hosts of Leptospira interrogans in Campeche, México. Rev Inst Med Trop Sao Paulo. 2015;57:181-3. https://doi.org/10.1590/S0036-46652015000200015
Zavala-Castro J, Zavala-Velázquez J, Walker D, Pérez-Osorio J, Peniche-Lara G. Severe human infection with Rickettsia felis associated with hepatitis in Yucatán, México. Int J Med Microbiol. 2009;299:529-33. https://doi.org/10.1016/j.ijmm.2009.03.002
Desvars A, Naze F, Benneveau A, Cardinale E, Michault A. Endemicity of leptospirosis in domestic and wild animal species from Reunion Island (Indian Ocean). Edemiol Infect. 2013;141:1154-65. https://doi.org/10.1017/S0950268812002075
Guernier V, Lagadec E, Cordonin C, Le Minter G, Gomard Y, Pagès F, et al. Human leptospirosis on Reunion Island, Indian Ocean: Are rodents the (only) ones to blame? PLoS Negl Trop Dis. 2016;10:e0004733. https://doi.org/10.1371/journal.pntd.0004733
Halliday JE, Knobel DL, Allan KJ, de C Bronsvoort BM, Handel I, Agwanda B, et al. Urban leptospirosis in Africa: A cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. Am J Trop Med Hyg. 2013;89:1095-102. https://doi.org/10.4269/ajtmh.13-0415
Mayer-Scholl A, Hammerl JA, Schmidt S, Ulrich RG, Pfeffer M, Woll D, et al. Leptospira spp. in rodents and shrews in Germany. Int J Environ Res Public Health. 2014;11:7562-74. https://doi.org/10.3390/ijerph110807562
Mgode GF, Mhamphil G, Katakweba A, Paemelaere E, Willekens N, Leirs H, et al. PCR detection of Leptospira DNA in rodents and insectivores from Tanzania. Belg J Zool. 2005;135:17-9.
Foronda P, Martín-Alonso A, Del Castillo-Figueruelo B, Feliu C, Gil H, Valladares B. Pathogenic Leptospira spp. in wild rodents, Canary Islands, Spain. Emerg Infect Dis. 2011;17:1781-2. https://doi.org/10.3201/eid1709.101470
De Faria MT, Calderwood MS, Athanazio DA, McBride AJ, Hartskeerl RA, Pereira MM, et al. Carriage of Leptospira interrogans among domestic rats from and urban setting highly endemic for leptospirosis in Brazil. Acta Trop. 2008;108:1-5. https://doi.org/10.1016/j.actatropica.2008.07.005
Yusti D, Arboleda M, Agudelo-Flórez P. Factores de riesgo sociales y ambientales relacionados con casos de leptospirosis de manejo ambulatorio y hospitalario, Turbo, Colombia. Biomédica. 2013;33:117-29. https://doi.org/10.7705/biomedica.v33i0.1457
Shelotto F, Hernández E, González S, Del Monte A, Ifran S, Flores K, et al. A ten-year follow-up of human leptospirosis in Uruguay: An unresolved health problem. Rev Inst Med Trop Sao Paulo. 2012;54:69-75. https://doi.org/10.1590/S0036-46652012000200003
Loan HK, van Cuong N, Takhampunya R, Kiet BT, Campbell J, Them LN, et al. How important are rats as vectors of leptospirosis in the Mekong Delta of Vietnam? Vector Borne Zoonotic Dis. 2015;15:56-65. https://doi.org/10.1089/vbz.2014.1613
Scialfa E, Bolpe J, Bardón JC, Ridao G, Gentile J, Gallicchio O. Isolation of Leptospira interrogans from suburban rats in Tandii, Buenos Aires, Argentina. Rev Argent Microbiol. 2010;42:126-128. https://doi.org/10.1590/S0325-75412010000200012
Verdasquera-Corcho D, Barroso-Corría J, Barreras-Suárez BA, Pérez-Rodríguez A, Pérez-Soler K, Obregón-Fuentes AM, et al. Factores asociados a la morbilidad por leptospirosis humana. Ciudad de La Habana, 2005-2006. Rev Panam Infectol. 2010;12:8-16.
Nájera S, Alvis N, Babilonia D, Álvarez L, Máttar S. Leptospirosis ocupacional en una región del Caribe colombiano. Salud Públ Méx. 2005;47:240-4.
Agudelo-Flórez P, Londoño AF, Quiroz VH, Ángel JC, Moreno N, Loaiza ET, et al. Prevalence of Leptospira spp. in urban rodents from a groceries trade center of Medellín, Colombia. Am J Trop Med Hyg. 2009;81:906-10. https://doi.org/10.4269/ajtmh.2009.09-0195
Treml F, Nepeřený J, Janová E, Banďouchová H, Pikula J. Prevalence of antibodies against leptospires in small mammals in relation to age, sex and season. ActaVer Brno. 2012;81:1097-102. https://doi.org/10.2754/avb201281020097
Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Díaz MM, Lovett MA, et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757-71.
https://doi.org/10.1016/S1473-3099(03)00830-2
Romero-Vivas CM, Thiry D, Rodríguez V, Calderón A, Arrieta G, Máttar S, et al. Molecular serovar characterization of Leptospira isolates from animals and water in Colombia. Biomédica. 2013;33:179-184. https://doi.org/10.7705/biomedica.v33i0.731
Some similar items:
- José Alejandro Martínez-Ibarra, Jorge Alejandro Martínez-Grant, Miguel Roberto Verdugo-Cervantes, Rafael Bustos-Saldaña, Benjamín Nogueda-Torres, Monitoring triatomid bug (Hemiptera: Reduviidae) presence by sentinel chicken coops in Southern Jalisco State, México , Biomedica: Vol. 30 No. 1 (2010)
- Gladys Acuña-González, Carlo E. Medina-Solís, Gerardo Maupomé, Mauricio Escoffie-Ramírez, Jesús Hernández-Romano, María de L. Márquez-Corona, Arturo J. Islas-Márquez, Juan J. Villalobos-Rodelo, Family history and socioeconomic risk factors for non-syndromic cleft lip and palate: A matched case-control study in a less developed country , Biomedica: Vol. 31 No. 3 (2011)
- Juan José Villalobos Rodelo, Carlo Eduardo Medina Solís, Nelly Molina Frechero, Ana Alicia Vallejos Sánchez, América Patricia Pontigo Loyola, José Luis Espinoza Beltrán, Dental caries in schoolchildren aged 6-12 years in Navolato, Sinaloa, México: experience, prevalence, severity and treatment needs. , Biomedica: Vol. 26 No. 2 (2006)
- Zinnia J. Molina-Garza, Lucio Galaviz-Silva, Pediculus capitis in schoolchildren of the urban area of Nuevo León, México: Analyses of associated factors , Biomedica: Vol. 37 No. 3 (2017)
- Daly Martínez-Ortiz, Marco Torres-Castro, Edgar Koyoc-Cardeña, Karina López, Alonso Panti-May, Iván Rodríguez-Vivas, Adriano Puc, Karla Dzul, Jorge Zavala-Castro, Anuar Medina-Barreiro, Juan Chablé-Santos, Pablo Manrique-Saide, Molecular evidence of Rickettsia typhi infection in dogs from a rural community in Yucatán, México , Biomedica: Vol. 36 (2016): Suplemento 1, Microbiología médica
- Analilia Solís-Hernández, Roger Iván Rodríguez-Vivas, María Dolores Esteve–Gassent, Sandra Luz Villegas-Pérez, Prevalence of Borrelia burgdorferi sensu lato in synanthropic rodents in two rural communities of Yucatán, México , Biomedica: Vol. 36 (2016): Suplemento 1, Microbiología médica
- Claudio Alberto Dávila, Ana Melisa Pardo, Suicide mortality in Colombia and México: Trends and impact between 2000 and 2013 , Biomedica: Vol. 36 No. 3 (2016)
- Rodrigo Adán Medina-Pinto, Roger Iván Rodríguez-Vivas, Manuel Emilio Bolio-González, Zoonotic intestinal nematodes in dogs from public parks in Yucatán, México , Biomedica: Vol. 38 No. 1 (2018)
- Lizbeth Díaz, Karen Covarrubias, Ángel Licón, Mixtli Astorga, Yaneth Moreno, José Alejandro Martínez, Biological parameters of Meccus phyllosomus phyllosomus (Burmeister), 1835, Triatoma recurva (Stål), 1868 (Hemiptera, Reduviidae) and their laboratory hybrids , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Jesús Delgado-De la Mora, Jesús David Licona-Enríquez, Marcia Leyva-Gastélum, David Delgado-De la Mora, Adela Rascón-Alcantar, Gerardo Álvarez-Hernández, A fatal case series of Rocky Mountain spotted fever in Sonora, México , Biomedica: Vol. 38 No. 1 (2018)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























