Nuclei ultrastructural changes of C6/36 cells infected with virus dengue type 2
Abstract
Introduction: Dengue virus replication has been considered mainly cytoplasmic, however, studies indicate that some flaviviruses may use the intranuclear pathway as part of the machinery that the virus uses to increase infection capacity in the host cell. This paper describes alterations at nuclear level in the cell infected with dengue, which are likely involved in the virus replication processes.
Objective: This paper addresses the ultrastructural observations of C6/36 cells of the Aedes albopictus mosquito infected with dengue virus type 2.
Materials and methods: C6/36 cells were infected in culture medium with the serum of a patient positively diagnosed for dengue 2. Subsequently, the cells were incubated for 10 days and the cytopathic effect was assessed. The cells were processed for immunofluorescence assays and transmission electron microscopy.
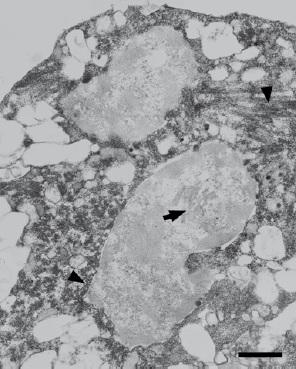
Results: The immunofluorescence assays confirmed the presence of viral protein E associated with cellular syncytia in the culture. In the ultrastructural study, the infected cells showed vesicular-tubular structures and dilated cisterns of the endoplasmic reticulum at the cytoplasmic level. Viral particles were found exclusively in cytoplasm localized within the vacuoles. Nuclei of cellular syncytia showed membrane structures arranged in a circular shape and, in some cases, these syncytia displayed lysis; in no case viral particles were observed at the nuclear level.
Conclusions: The ultrastructural alterations of nuclei in cells infected with the dengue virus using electron microscopy techniques had not been reported before, as far as we know. It is likely that such modifications are associated with replicative processes at an intranuclear level as an alternate replication mechanism.
Downloads
References
Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37-43. https://doi.org/10.1099/0022-1317-70-1-37
del Ángel RM. Entrada del virus del dengue: moléculas que pueden modular la patogenia viral. Cinvestad. 2006;25:38-43.
Nascimento D, Castro AR, Froes IB, Bigaton G, Oliveira EC, Dal Fabbro MF, et al. Clinical and laboratory findings in patients with dengue associated with hepatopathy. Rev Soc Bras Med Trop. 2011;44:674-7. https://doi.org/10.1590/S0037-86822011005000061
Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100:608-14. https://doi.org/10.1016/j.trstmh.2005.10.007
Ling LM, Wilder-Smith A, Leo YS. Fulminant hepatitis in dengue haemorrhagic fever. J Clin Virol. 2007;38:265-8. https://doi.org/10.1016/j.jcv.2006.12.011
Bartenschlager R, Miller S. Molecular aspects of dengue virus replication. Future Microbiol. 2008;3:155-65. https://doi.org/10.2217/17460913.3.2.155
Gubler DJ, Ooi EE, Vasudevan S, Farrar J. Dengue and dengue hemorrhagic fever. Second edition. Singapore: CABI; 2014. p. 337. https://doi.org/10.1079/9781845939649.0000
Kumar A, Buhler S, Selisko B, Davidson A, Mulder K, Canard B, et al. Nuclear localization of dengue virus nonstructural protein 5 does not strictly correlate with efficient viral RNA replication and inhibition of type I interferon signaling. J Virol. 2013;87:4545-57. https://doi.org/10.1128/JVI.03083-12
Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418-31. https://doi.org/10.1128/JVI.01257-06
Figueiredo LT. Uso de células de Aedes albopictus C6/36 na propagação e classificação de arbovírus das famílias Togaviridae, Flaviviridae, Bunyaviridae e Rhabdoviridae. Rev Soc Bras Med Trop.1990;23:13-8. https://doi.org/10.1590/S0037-86821990000100003
Batista WC, Tavares Gda S, Vieira DS, Honda ER, Pereira SS, Tada MS. Notification of the first isolation of Cacipacore virus in a human in the State of Rondonia, Brazil. Rev Soc Bras Med Trop. 2011;44:528-30. https://doi.org/10.1590/S0037-86822011000400028
Grief C, Galler R, Cortés LM, Barth OM. Intracellular localisation of dengue-2 RNA in mosquito cell culture using electron microscopic in situ hybridisation. Arch Virol. 1997;142:2347-57.
Barth OM. Replication of dengue viruses in mosquito cell cultures--A model from ultrastructural observations. Mem Inst Oswaldo Cruz. 1992;87:565-74. https://doi.org/10.1590/S0074-02761992000400017
Castañeda NY, Castellanos JE, Zapata AC, Bello F. Línea celular de Aedes aegypti (DIPTERA: CULICIDAE) AEGY28 refractaria a la infección con los virus dengue 2 y fiebre amarilla. Acta Biológica Colombiana. 2007;12:47-58.
World Health Organization. Guidelines for good clinical practice (GCP) for trials on pharmaceutical products. WHO Technical Report Series. 1995;850:97-137.
Barth OM, De Castro-Côrtes LM. Morphology of dengue virus induced cell syncytia. Acta Microscopica. 1997;6:1-8.
Ko KK, Igarashi A, Fukai K. Electron microscopic observations on Aedes albopictus cells infected with dengue viruses. Arch Virol.1979;62:41-52.
Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J Virol. 2014;88:4687-97. https://doi.org/10.1128/JVI.00118-14
Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, et al. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365-75. https://doi.org/10.1016/j.chom.2009.03.007
Cardiff RD, Russ SB, Brandt WE, Russell PK. Cytological localization of Dengue-2 antigens: An immunological study with ultrastructural correlation. Infect Immun.1973;7:809-16.
Stohlman SA, Wisseman CL Jr., Eylar OR, Silverman DJ. Dengue virus-induced modifications of host cell membranes. J Virol. 1975;16:1017-26.
Marianneau P, Steffan AM, Royer C, Drouet MT, Kirn A, Deubel V. Differing infection patterns of dengue and yellow fever viruses in a human hepatoma cell line. J Infect Dis. 1998;178:1270-8. https://doi.org/10.1086/314466
Matsumura T, Stollar V, Schlesinger RW. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971;46:344-55. https://doi.org/10.1016/0042-6822(71)90036-5
Demsey A, Steere RL, Brandt WE, Veltri BJ. Morphology and development of dengue-2 virus employing the freezefracture and thin-section techniques. J Ultrastruct Res. 1974;46:103-16.
Raes H, Braeckman BP, Criel GR, Rzeznik U, Vanfleteren JR. Copper induces apoptosis in Aedes C6/36 cells. J Exp Zool. 2000;286:1-12. https://doi.org/10.1002/(SICI)1097-010X(20000101)286
den Boon JA, Díaz A, Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8:77-85. https://doi.org/10.1016/j.chom.2010.06.010
Salonen A, Ahola T, Kaariainen L. Viral RNA replication in association with cellular membranes. Curr Top Microbiol Immunol. 2005;285:139-73.
Miller S, Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363-74. https://doi.org/10.1038/nrmicro1890
Romero-Brey I, Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6:2826-57. https://doi.org/10.3390/v6072826
Whittaker GR, Kann M, Helenius A. Viral entry into the nucleus. Annu Rev Cell Dev Biol. 2000;16:627-51. https://doi.org/10.1146/annurev.cellbio.16.1.627
Hiscox JA. The interaction of animal cytoplasmic RNA viruses with the nucleus to facilitate replication. Virus Res. 2003;95:13-22. https://doi.org/10.1016/S0168-1702(03)00160-6
Uchil PD, Satchidanandam V. Characterization of RNA synthesis, replication mechanism, and in vitro RNA-dependent RNA polymerase activity of japanese encephalitis virus. Virology. 2003;307:358-71. https://doi.org/10.1016/S0042-6822(02)00130-7
Zebovitz E, Leong JK, Doughty SC. Involvement of the host cell nuclear envelope membranes in the replication of Japanese encephalitis virus. Infect Immun. 1974;10:204-11.
Uchil PD, Kumar AV, Satchidanandam V. Nuclear localization of flavivirus RNA synthesis in infected cells. J Virol. 2006;80:5451-64. https://doi.org/10.1128/JVI.01982-05
Kos KA, Osborne BA, Goldsby RA. Inhibition of group B arbovirus antigen production and replication in cells enucleated with cytochalasin B. J Virol. 1975;15:913-7.
Kapoor M, Zhang L, Ramachandra M, Kusukawa J, Ebner KE, Padmanabhan R. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J Biol Chem. 1995;270:19100-6. https://doi.org/10.1074/jbc.270.32.19100
Weidman MK, Sharma R, Raychaudhuri S, Kundu P, Tsai W, Dasgupta A. The interaction of cytoplasmic RNA viruses with the nucleus. Virus Res. 2003;95:75-85. https://doi.org/10.1016/S0168-1702(03)00164-3
Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99:301-6. https://doi.org/10.1016/j.antiviral.2013.06.002
Sangiambut S, Keelapang P, Aaskov J, Puttikhunt C, Kasinrerk W, Malasit P, et al. Multiple regions in dengue virus capsid protein contribute to nuclear localization during virus infection. J Gen Virol. 2008;89:1254-64. https://doi.org/10.1099/vir.0.83264-0
Wang SH, Syu WJ, Huang KJ, Lei HY, Yao CW, King CC, et al. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J Gen Virol. 2002;83:3093-102. https://doi.org/10.1099/0022-1317-83-12-3093
Netsawang J, Noisakran S, Puttikhunt C, Kasinrerk W, Wongwiwat W, Malasit P, et al. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 2010;147:275-83. https://doi.org/10.1016/j.virusres.2009.11.012
Buckley A, Gould EA. Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with Zika or Langat virus. J Gen Virol. 1988;69:1913-20. https://doi.org/10.1099/0022-1317-69-8-1913
Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974-84. https://doi.org/10.1128/JVI.76.12.5974-5984.2002
Miller S, Kastner S, Krijnse-Locker J, Buhler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873-82. https://doi.org/10.1074/jbc.M609919200
Sarmiento L, Rengifo AC, Rivera J, Neira M, Parra E, Méndez J, et al. Glucógeno hepático en dengue severo: análisis histopatológico. Infectio. 2013;17:172-6. https://doi.org/10.1016/S0123-9392(13)70728-8
Thakur P, Lamoke F, Chaffin JM, Bartoli M, Lee JR, Duncan MB. Dysplastic hepatocytes develop nuclear inclusions in a mouse model of viral hepatitis. PloS One. 2014;9:e99872. https://doi.org/10.1371/journal.pone.0099872
Pryor MJ, Rawlinson SM, Butcher RE, Barton CL, Waterhouse TA, Vasudevan SG, et al. Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta-recognized nuclear localization sequences is integral to viral infection. Traffic. 2007;8:795-807. https://doi.org/10.1111/j.1600-0854.2007.00579.x
Diwaker D, Mishra KP, Ganju L, Singh SB. Dengue virus non-structural 1 protein interacts with heterogeneous nuclear ribonucleoprotein H in human monocytic cells.
Asian Pac J Trop Med.2016;9:112-8. https://doi.org/10.1016/j.apjtm.2016.01.015
Tadano M, Makino Y, Fukunaga T, Okuno Y, Fukai K. Detection of dengue 4 virus core protein in the nucleus. I. A monoclonal antibody to dengue 4 virus reacts with the antigen in the nucleus and cytoplasm. J Gen Virol. 1989;70:1409-15. https://doi.org/10.1099/0022-1317-70-6-1409
Bulich R, Aaskov JG. Nuclear localization of dengue 2 virus core protein detected with monoclonal antibodies. J Gen Virol. 1992;73:2999-3003. https://doi.org/10.1099/0022-1317-73-11-2999
Some similar items:
- Marcela Conde, Lorena I. Orjuela, Cesar Augusto Castellanos, Manuela Herrera-Varela, Susana Licastro, Martha L. Quiñones, Insecticide susceptibility evaluation in Aedes aegypti populations of Caldas, Colombia, in 2007 and 2011 , Biomedica: Vol. 35 No. 1 (2015)
- Celeny Ortiz, Guillermo L. Rúa-Uribe, Carlos A. Rojas, Knowledge, practices and entomological aspects of dengue in Medellín, Colombia: A comparative study of neighborhoods with high and low incidence , Biomedica: Vol. 38 No. Sup. 2 (2018): Suplemento 2, Medicina tropical
- José Joaquín Carvajal, Nildimar Alves Honorio, Silvia Patricia Díaz, Edinso Rafael Ruiz, Jimmy Asprilla, Susanne Ardila, Gabriel Parra-Henao, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the municipality of Istmina, Chocó, Colombia , Biomedica: Vol. 36 No. 3 (2016)
- Lorenzo Cáceres , Cipriano Ayarza, Damaris Bernal, Evaluation of the biological efficacy and susceptibility in Aedes aegypti to the pyrethroid insecticides deltamethrin and cyfluthrin during the Zika virus outbreak in Kuna Yala, Panama , Biomedica: Vol. 43 No. 2 (2023)
- María Elena Cuéllar-Jiménez, Olga Lucía Velásquez-Escobar, Ranulfo González-Obando, Carlos Andrés Morales-Reichmann, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia , Biomedica: Vol. 27 No. 2 (2007)
- Jorge R. Rey, Philip Lounibos, Ecology of Aedes aegypti and Aedes albopictus in the Americas and disease transmission , Biomedica: Vol. 35 No. 2 (2015)
- Raúl A. Rojo-Ospina, Marcela Quimbayo-Forero, Arley Calle-Tobón, Sindy C. Bedoya-Patiño, Maribel Gómez, Astrid Ramírez, Johnny Sánchez, Juan F. Silva-Alzate, Carlos J. Montes-Zuluaga, Jorge M. Cadavid, Enrique A. Henao-Correa, Integrated vector management program in the framework of the COVID-19 pandemic in Medellin, Colombia , Biomedica: Vol. 43 No. 1 (2023)
- Wilber Gómez-Vargas, Kelly Valencia-Jiménez, Guillermo Correa-Londoño, Faiber Jaramillo-Yepes, Novel larvicide tablets of Bacillus thuringiensis var. israelensis: Assessment of larvicidal effect on Aedes aegypti (Diptera: Culicidae) in Colombia , Biomedica: Vol. 38 No. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Mabel Carabalí, Clara Beatriz Ocampo, María Eugenia Toledo, Lyda Osorio, Mass communication of dengue surveillance data: effect of an intervention in Guadalajara de Buga, Colombia , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Sandy Milena Caldera, María Cristina Jaramillo, Suljey Cochero, Alveiro Pérez-Doria, Eduar Elías Bejarano, Genetic differences between populations of Aedes aegypti from municipalities in northern Colombia, with high and low dengue incidence , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























