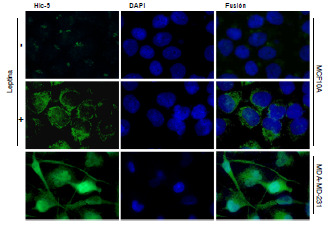
Leptin induced Hic-5 expression and actin puncta formation by the FAK/Src-dependent pathway in MCF10A mammary epithelial cells.
Abstract
Introduction: Leptin is a hormone secreted by adipocytes that has been associated with the epithelial-mesenchymal transition (EMT). Additionally, leptin promotes the migration and invasion of mammary epithelial cells through the activation of FAK and Src kinases, which are part of a regulatory complex of signaling pathways that promotes the expression of proteins related to the formation of proteolytic structures involved in the invasion and progression of cancer. Recently, overexpression and activation of Hic-5 during the EMT have been shown to induce the formation of actin puncta; these structures are indicative of the formation and functionality of invadopodia, which promote the local degradation of extracellular matrix components and cancer metastasis.
Objective: To evaluate the role of FAK and Src kinases in the expression of Hic-5 during the epithelial-mesenchymal transition induced by leptin in MCF10A cells.
Materials and methods: We used specific inhibitors of FAK (PF-573228) and Src (PP2) to evaluate Hic-5 expression and subcellular localization by Western blot and immunofluorescence assays and to investigate the formation of actin puncta by epifluorescence in MCF10A cells stimulated with leptin.
Results: Leptin induced an increase in Hic-5 expression and the formation of actin puncta. Pretreatment with inhibitors of FAK (PF-573228) and Src (PP2) promoted a decrease in Hic-5 expression and actin puncta formation in the non-tumorigenic mammary epithelial cell line MCF10A.
Conclusion: In MCF10A cells, leptin-induced Hic-5 expression and perinuclear localization, as well as the formation of actin puncta through a mechanism dependent on the kinase activity of FAK and Src.
Downloads
References
Friedman J, Halaas J. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763-70. https://doi.org/10.1038/27376
Sánchez JC. Perfil fisiológico de la leptina. Colombia Med. 2005;36:50-9.
Grossmann M, Ray A, Nkhata K, Malakhov D, Regozina O, Dogan S, et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641-53. https://doi.org/10.1007/s10555-010-9252-1
González-Fernández J, Ugalde-Ovares CE. La glándula mamaria, embriología, histología, anatomía y una de sus principales patologías, el cáncer de mama. Revista Médica de Costa Rica y Centroamérica. 2012;602:317-20.
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2014;64:252-71. https://doi.org/10.3322/caac.21235
Lozano-Ascencio R, Gómez-Dantés H, Lewis S, Torres-Sánchez L, López-Carrillo L. Tendencias del cáncer de mama en América Latina y El Caribe. Salud Pública Mex. 2009;51:147-56.
Yuan HJ, Sun KW, Yu K. Leptin promotes the proliferation and migration of human breast cancer through the extracellular-signal regulated kinase pathway. Mol Med Rep. 2014;9:350-4. https://doi.org/10.3892mmr.2013.1786
Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 proteindependent pathways. J Biol Chem. 2012;287:8598-612. https://doi.org/10.1074/jbc.M111.322800
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-8. https://doi.org/10.1172/JCI39104
Maier HJ, Wirth T, Beug H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers. 2010;2:2058-83. http://dx.doi.org/10.3390/cancers2042058
Foroni C, Broggini M, Generali D, Damia G. Epithelial–mesenchymal transition and breast cancer: Role, molecular mechanisms and clinical impact. Cancer Treat Rev. 2012;38:689-97. https://doi.org/10.1016/j.ctrv.2011.11.001
Nantajit D, Lin D, Li JJ. The network of epithelial-mesenchymal transition: Potential new targets for tumor resistance. J Cancer Res Clin Oncol. 2014;141:1697-713. https://doi.org/10.1007/s00432-014-1840-y
Avtanski DB, Nagalingam A, Bonner MY, Arbiser JL, Saxena NK, Sharma D. Honokiol activates LKB1-miR-34a axis and antagonizes the oncogenic actions of leptin in breast cancer. Oncotarget. 2015;6:29947-62. https://doi.org/10.18632/oncotarget.4937
Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12-22. https://doi.org/10.1002/jcp.20472
Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, et al. Activation of IL-8 via PI3K/Aktdependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16:1220-30. https://doi.org/10.1080/15384047.2015.1056409
Beaty B, Condeelis J. Digging a little deeper: The stages of invadopodium formation and maturation. Eur J Cell Biol. 2014;93:438-44. https://doi.org/ 10.1016/j.ejcb.2014.07.003
Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421-37. https://doi.org/10.1083/jcb.201108143
Shibanuma M, Mochizuki E, Maniwa R, Mashimo J, Nishiya N, Imai S, et al. Induction of senescence-like phenotypes by forced expression of hic-5, which encodes a novel LIM motif protein, in immortalized human fibroblasts. Mol Cell Biol. 1997;17:1224-35. https://doi.org/10.1128/MCB.17.3.1224
Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci. 1999;112:181-90.
Varney SD, Betts CB, Zheng R, Wu L, Hinz B, Zhou J, et al. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J Cell Sci. 2016;129:774-87. https://doi.org/10.1242/jcs.170589
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56-68. https://doi.org/10.1038/nrm1549
Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke CI, Guelcher SA, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295-9. https://doi.org/10.1016/j.cub.2008.07.090
Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: A role for Src family kinases. Mol Cell Biol. 1995;15:954-63.
Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adh Migr. 2009;3:288-92. https://doi.org/10.4161/cam.3.3.8888
Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597-606. https://doi.org/10.1016/j.ceb.2011.04.002
Villanueva-Duque A, Zúñiga-Eulogio MD, Dena-Beltrán J, Castañeda-Saucedo E, Calixto-Gálvez M, Mendoza-Catalán M, et al. Leptin induces partial epithelial-mesenchymal transition in a FAK-ERK dependent pathway in MCF10A mammary non-tumorigenic cells. Int J Clin Exp Pathol. 2017;10:10334-42.
Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578-82. https://doi.org/10.1038/sj.bjc.6604913
Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447-53. https://doi.org/10.1158/1078-0432.CCR-05-1913
Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2010;22:327-41. https://doi.org/10.1091/mbc.E10-09-0790
Sheta R, Wang ZQ, Bachvarova M, Plante M, Gregoire J, Renaud MC, et al. Hic-5 regulates epithelial to mesenchymal transition in ovarian cancer cells in a TGFβ1-independent manner. Oncotarget. 2017;8:82506-30. https://doi.org/10.18632/oncotarget.19714
Wu JR, Hu CT, You RI, Pan SM, Cheng CC, Lee MC, et al. Hydrogen peroxide inducible clone-5 mediates reactive oxygen species signaling for hepatocellular carcinoma progression. Oncotarget. 2015;6:32526-44. https://doi.org/10.18632/oncotarget.5322
Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: Organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11:802-14. https://doi.org/10.1038/nrm2996
Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression of the focal adhesion kinase (p125 FAK) in invasive human tumors. Cancer Res. 1995;55:2752-6.
Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to RAS pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786-91. https://doi.org/10.1038/372786a0
Nishiya N, Tachibana K, Shibanuma M, Mashimo JI, Nose K. Hic-5-reduced cell spreading on fibronectin: Competitive effects between paxillin and hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21:5332-45. https://doi.org/10.1128/MCB.21.16.5332-5345.2001
Ligthfoot HM, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, et al. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88:109-16. https://doi.org/10.1007/s10549-004-1022-8
Chambers AF, Groom AC, Macdonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563-72. https://doi.org/10.1038/nrc865
Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src’s hold over actin and cell adhesion. Nat Rev Mol Cell Biol. 2002;3:233-45. https://doi.org/10.1038/nrm779
Yeatman TJ. A renaissance for Src. Nat Rev Cancer. 2004;4:470-80. https://doi.org/10.1038/nrc1366
Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geer zema JG, Hennipman A, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol. 1996;180:383-8. https://doi.org/10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N
Elsberger B, Fullerton R, Zino S, Jordan F, Mitchell TJ, Brunton VG, et al. Breast cancer patients’ clinical outcome measures are associated with Src kinase family member expression. Br J Cancer. 2010;103:899-909. https://doi.org/10.1038/sj.bjc.6605829
Kanomata N, Kurebayashi J, Kozuka Y, Sonoo H, Moriya T. Clinicopathological significance of Y416Src and Y527Src expression in breast cancer. J Clin Pathol. 2011;64:578-58. https://doi.org/10.1136/jclinpath-2011-200042
Jiang L, Li Z, Rui L. Leptin stimulates both jak2-dependent and jak2-independent signaling pathways. J Biol Chem. 2008;283:28066-73. https://doi.org/10.1074/jbc.M805545200
Hanks SK, Ryzhova L, Shin NY, Brábek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:982-96.
Serrels B, Serrels A, Brunton VG, Holt M, Mclean GW, Gray CH, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046-56. https://doi.org/10.1038/ncb1626
Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci USA. 2007;104:11933-8. https://doi.org/10.1073/pnas.0701077104
Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: The role of the N-WASP–Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441-52. https://doi.org/10.1083/jcb.200407076
Some similar items:
- Elpidia Poveda, Pilar Trujillo, Francisco Ruiz, Elizabeth Lopez, Glucose and insulin levels in Wistar rats submitted to high fat diet and treatment with mimetic leptin peptides , Biomedica: Vol. 28 No. 1 (2008)
- Oscar F. Herrán, María F. Ardila, Martha P. Rojas, Gustavo A. Hernández, Design of dietary questionnaires to study the relationships between diet and cancer prevalence in Colombia , Biomedica: Vol. 30 No. 1 (2010)
- Ismael Reyes, Raj Tiwari, Jan Geliebter, Niradiz Reyes, DNA microarray analysis reveals metastasis-associated genes in rat prostate cancer cell lines , Biomedica: Vol. 27 No. 2 (2007)
- Juan Carlos Herrera, Luis Fernando Isaza, José Luis Ramírez, Gonzalo Vásquez, Carlos Mario Muñetón, Detection of chromosome 17 aneuplody and TP53 gene deletion in a broad variety of solid tumors by dual-color fluorescence in situ hybridization (FISH) , Biomedica: Vol. 30 No. 3 (2010)
- Ricardo Cendales, Constanza Pardo, Claudia Uribe, Guillermo López, María Clara Yépez, Luis Eduardo Bravo, Data quality at population-based cancer registries in Colombia , Biomedica: Vol. 32 No. 4 (2012)
- Sonia Isabel Cuervo, Ricardo Sánchez, Julio César Gómez-Rincón, Cielo Almenares, Juan Pablo Osorio, María José Vargas, Behavior of carbapenemase-producing Klebsiella pneumoniae cases in cancer patients at a third level hospital in Bogotá, D.C. , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Clara Andrea Rincón-Cortés, Edgar Antonio Reyes-Montaño, Nohora Angélica Vega-Castro, Partial purification of peptides present in the Tityus macrochirus (Buthidae) scorpion venom and preliminary assessment of their cytotoxicity , Biomedica: Vol. 37 No. 2 (2017)
- Esther de Vries, María Ximena Meneses, Marion Piñeros, Years of life lost as a measure of cancer burden in Colombia, 1997-2012 , Biomedica: Vol. 36 No. 4 (2016)
- Islendy Noreña, Myriam Patricia Pardo, Ismena Mockus, Serum adipokine levels and insulin resistance in the first trimester of pregnancy in adolescents and their relationship with neonatal weight , Biomedica: Vol. 38 No. 3 (2018)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























