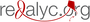In vitro effectivity of three approved drugs and their synergistic interaction against Leishmania infantum
Abstract
Introduction: Leishmaniasis remains one of the neglected tropical diseases. Repurposing existing drugs has proven to be successful for treating neglected tropical diseases while combination therapy is a strategic alternative for the treatment of infectious diseases.
Auranofin, lopinavir/ritonavir, and sorafenib are FDA approved drugs used in the treatment of diverse diseases by acting on different essential biological enzymes.
Objective: To evaluate the effects of monotherapy and combined therapies with the three drugs against Leishmania infantum.
Materials and methods: We compared the leishmanicidal effects of the three drugs on promastigotes in vitro as regards the parasite count, the drug concentration providing a half-maximal response, and the ultrastructural changes of the parasite. We determined the fractional inhibitory concentration index of combined drugs in two ways, as well as the activity of the three drugs together to establish their synergetic effect.
Results: The monotherapy with the three drugs was effective with auranofin showing the best leishmanicidal effect (EC50=1.5 μM), whereas sorafinib reduced parasite growth at EC50=2.5 μM. The scanning electron microscopy of promastigotes from all treated media showed distortion in the shape with loss of flagella and bleb formation. Acidocalcinosis was evident by transmission electron microscopy with all treatments suggesting apoptosis. Treatment with lopinavir/ritonavir showed signs of autophagy. The two-way combination of the drugs led to additive interactions while the combination of the three drugs showed synergistic action.
Conclusion: Each drug when used as monotherapy against Leishmania spp. was effective, but the combination therapy was more effective than the individual drugs due to the additive or synergistic effects.
Downloads
References
WHO Expert Committee on the Control of the Leishmaniases and World Health Organization. Control of the leishmaniases: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March, 2010. Geneva: World Health Organization; 2010.
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. https://doi.org/10.1371/journal.pone.0035671
Abou-El-Naga IF. Demographic, socioeconomic and environmental changes affecting the circulation of neglected tropical diseases in Egypt. Asian Pac J Trop Med. 2015;8:881-8. https://doi.org/10.1016/j.apjtm.2015.10.015
Zijlstra EE. PKDL and other dermal lesions in HIV co-infected patients with Leishmaniasis: Review of clinical presentation in relation to immune responses. PLoS Negl Trop Dis. 2014;20:8:e3258. https://doi.org/10.1371/journal.pntd.0003258
Grogl M, Hickman M, Ellis W, Hudson T, Lazo JS, Sharlow ER, et al. Drug discovery algorithm for cutaneous leishmaniasis. Am J Trop Med Hyg. 2013;88:216-21. https://doi.org/10.4269/ajtmh.11-0812
Amato VS, Tuon FF, Bacha HA, Neto VA, Nicodemo AC. Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Trop. 2008;105:1-9. https://doi.org/10.1016/j.actatropica.2007.08.003
Singh N, Kumar M, Singh RK. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012;5:485-97. https://doi.org/10.1016/S1995-7645(12)60084-4
Ekins S, Williams AJ. Finding promiscuous old drugs for new uses. Pharm Res. 2011;28:1785-91. https://doi.org/10.1007/s11095-011-0486-6
Planer JD, Hulverson MA, Arif JA, Ranade RM, Don R, Buckner FS. Synergy testing of FDA approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl Trop Dis. 2014;8:e2977. https://doi.org/10.1371/journal.pntd.0002977
Das P, Alam MN, Paik D, Karmakar K, De T, Chakraborti T. Protease inhibitors in potential drug development for leishmaniasis. Indian J Biochem Biophys. 2013;50:363-76.
Saha AK, Mukherjee T, Bhaduri A. Mechanism of action of amphotericin B on Leishmania donovani promastigotes. Mol Biochem Parasitol. 1986;19:195-200. https://doi.org/10.1016/0166-6851(86)90001-0
Alvar J, Croft S, Olliaro P. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol. 2006;61:223-74. https://doi.org/10.1016/S0065-308X(05)61006-8
Rigobello MP, Scutari G, Boscolo R, Bindoli A. Induction of mitochondrial permeability transition by auranofin, a gold(I)–phosphine derivative. Br J Pharmacol. 2002;136:1162-8. https://doi.org/10.1038/sj.bjp.0704823
Ilari A, Baiocco P, Messori L, Fiorillo A, Boffi A, Gramiccia M, et al. A gold-containing drug against parasitic polyamine metabolism: The X-ray structure of trypanothione reductase from Leishmania infantum in complex with auranofin reveals a dual mechanism of enzyme inhibition. Amino Acids. 2012;42:803-11. https://doi.org/10.1007/s00726-011-0997-9
Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023-33. https://doi.org/10.2147/TCRM.S3285
Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63:769-802. https://doi.org/10.2165/00003495-200363080-00004
Klemba M, Goldberg DE. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:183-91. https://doi.org/10.1016/j.molbiopara.2005.05.015
Derouin F, Santillana-Hayat M. Anti-Toxoplasma activities drugs and interactions with pyrimethamine and sulfadiazine in vitro. Antimicrob Agents Chemother. 2000;44:2575-7. https://doi.org/10.1128/AAC.44.9.2575-2577.2000
Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005;49:2983-5. https://doi.org/10.1128/AAC.49.7.2983-2985.2005
Savoia D, Allice T, Tovo PA. Anti-leishmanial activity of HIV protease inhibitors. Int J Antimicrob Agents. 2005;26:92-4. https://doi.org/10.1016/j.ijantimicag.2005.04.003
Santos LO, Vitório BS, Branquinha MH, Pedroso e Silva CM, Santos AL, d’Avila-Levy CM. Nelfinavir is effective in inhibiting the multiplication and aspartic peptidase activity of Leishmania species, including strains obtained from HIV-positive patients. J Antimicrob Chemother. 2013;68:348-53. https://doi.org/10.1093/jac/dks410
Pozio E. Highly active antiretroviral therapy and opportunistic protozoan infections. Parassitologia. 2004;46:83-9.
Trudel N, Garg R, Messier N, Sundar S, Ouellette M, Tremblay MJ. Intracellular survival of Leishmania species that cause visceral leishmaniasis is significantly reduced by HIV-1 protease inhibitors. J Infect Dis. 2008;198:1292-9. https://doi.org/10.1086/592280
Santos LO, Marinho FA, Altoé EF, Vitório BS, Alves CR, Britto C, et al. HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonensis. PLoS One. 2009;4:e4918. https://doi.org/10.1371/journal.pone.0004918
Demarchi IG, Silveira TG, Ferreira IC, Lonardoni MV. Effect of HIV protease inhibitors on New World Leishmania. Parasitol Int. 2012;61:538-44. https://doi.org/10.1016/j.parint.2012.04.006
Sanderson L, Yardley V, Croft SL. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. J Antimicrob Chemother. 2014;69:1888-91. https://doi.org/10.1093/jac/dku069
Cleghorn LA, Woodland A, Collie IT, Torrie LS, Norcross N, Luksch T, et al. Identification of inhibitors of the Leishmania cdc2-related protein kinase CRK3. Chem Med Chem. 2011;6:2214-24. https://doi.org/10.1002/cmdc.201100344
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127-32. https://doi.org/10.1038/nbt1358
Goldberg DE, Siliciano RF, Jacobs WR. Outwitting evolution: Fighting drug-resistant TB, malaria, and HIV. Cell. 2012;148:1271-83. https://doi.org/10.1016/j.cell.2012.02.021
Ménez C, Buyse M, Besnard M. Interaction between miltefosine and amphotericin B: Consequences for their activities towards intestinal epithelial cells and Leishmania donovani promastigotes in vitro. Antimicrob Agents Chemother. 2006;50:3793-800. https://doi.org/10.1128/AAC.00837-06
Seifert K, Munday J, Syeda T. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J Antimicrob Chemother. 2011;66:850-4. https://doi.org/10.1093/jac/dkq542
de Morais-Teixeira E, Gallupo MK, Rodrigues LF, Romanha AJ, Rabello A. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three New World Leishmania species. J Antimicrob Chemother. 2014;69:150-4. https://doi.org/10.1093/jac/dkt318
Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175-96. https://doi.org/10.1093/bmb/lds031
McCartry-Burke C, Bates PA, Dwyer DM. Leishmania donovani: Use of two different commercially available chemically defined media for the continuous in vitro cultivation of promastigotes. Exp Parasitol. 1991;73:385-7. https://doi.org/10.1016/0014-4894(91)90112-A
Garcia LS. Parasite recovery: Culture methods, animal inoculation and xenodiagnosis. In: García LS, editor. Diagnostic Medical Parasitology. 5th edition. Washington, USA: American Society for Microbiology; 2007. p. 927-8.
Fivelman QL, Adagu IS, Warhurst DC. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:4097-102. https://doi.org/10.1128/AAC.48.11.4097-4102.2004
Klainer AS, Betsch CJ. Scanning-beam electron microscopy of selected microorganisms. J Infect Dis. 1970;121:339-43. https://doi.org/10.1093/infdis/121.3.339
Bozzola JJ. Conventional specimen preparation techniques for transmission electron microscopy of cultured cells. Methods Mol Biol. 2007;369:1-18. https://doi.org/10.1007/978-1-59745-294-6_1
Chan YH. Biostatistics 102: Quantitative data- parametric & non-parametric tests. Singapore Med J. 2003;44:391-6.
Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, Nam J, et al. Anti-leishmanial highthroughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis. 2010;4:e675. https://doi.org/10.1371/journal.pntd.0000675
Gazanion E, Vergnes B, Seveno M. In vitro activity of nicotinamide/anti-leishmanial drug combinations. Parasitol Int. 2011;60:19-24. https://doi.org/10.1016/j.parint.2010.09.005
Crowther GJ, Shanmugam D, Carmona SJ, Doyle MA, Hertz-Fowler C, Berriman M, et al. Identification of attractive drug targets in neglected-disease pathogens using an in silico approach. PLoS Negl Trop Dis. 2010;4:e804. https://doi.org/10.1371/journal.pntd.0000804
Valdivieso E, Dagger F, Rascon A. Leishmania mexicana: Identification and characterization of an aspartyl proteinase activity. Exp Parasitol. 2007;116:77-82. https://doi.org/10.1016/j.exppara.2006.10.006
Alves ÉA, de Miranda MG, Borges TK, Magalhães KG, Muniz-Junqueira MI. Anti-HIV drugs, lopinavir/ritonavir and atazanavir, modulate innate immune response triggered by Leishmania in macrophages: The role of NF-κB and PPAR-γ. Int Immunopharmacol. 2015;24:314-24. https://doi.org/10.1016/j.intimp
Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648-57. https://doi.org/10.1111/jcmm.13008
Mesquita JT, Tempone AG, Reimão JQ. Combination therapy with nitazoxanide and amphotericin B, glucantime, miltefosine and sitamaquine against Leishmania infantum intracellular amastigotes. Acta Trop. 2014;130:112-6. https://doi.org/10.1016/j.actatropica.2013.11.003
Butcher EC. Can cell systems biology rescue drug discovery? Nat Rev Drug Discov. 2005;4:461-7. https://doi.org/10.1038/nrd1754
Lewis MG, Da Fonseca S, Chomont N, Palamara AT, Tardugno M, Mai A, et al. Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS. 2011;25:1347-56. https://doi.org/10.1097/QAD.0b013e328347bd77
Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111-26. https://doi.org/10.1128/CMR.19.1.111-126.2006
Olliaro PL. Drug combinations for visceral leishmaniasis. Curr Opin Infect Dis. 2010;23:595-602. https://doi.org/10.1097/QCO.0b013e32833fca9d
Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, et al. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci USA. 2003;100:7977-82. https://doi.org/10.1073/pnas.1337088100
Sharlow ER, Leimgruber S, Murray S, Lira A, Sciotti RJ, Hickman M, et al. Auranofin is an apoptosis-simulating agent with in vitro and in vivo anti-leishmanial activity. ACS Chem Biol. 2014;21:663-72. https://doi.org/10.1021/cb400800q
González-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquère S, et al. Apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091-102. https://doi.org/10.1242/jcs.02447
Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663-9. https://doi.org/10.1016/j.ceb.2004.09.011
Some similar items:
- Iveth J. González, Metacaspases and their role in the life cycle of human protozoan parasites , Biomedica: Vol. 29 No. 3 (2009)
- María Elena Maldonado, Souad Bousserouel, Francine Gossé, Annelise Lobstein, Francis Raul, Implication of NF-κB and p53 in the expression of TRAIL-death receptors and apoptosis by apple procyanidins in human metastatic SW620 cells , Biomedica: Vol. 30 No. 4 (2010)
- María Teresa Rugeles, Paula A. Velilla, Carlos J. Montoya, Mechanisms of human natural resistance to HIV: A summary of ten years of research in the Colombian population , Biomedica: Vol. 31 No. 2 (2011)
- Jairo E. Mateus, Wilfredo Valdivieso, Indira P. Hernández, Fernando Martínez, Edgar Páez, Patricia Escobar, Cell accumulation and antileishmanial effect of exogenous and endogenous protoporphyrin IX after photodynamic treatment , Biomedica: Vol. 34 No. 4 (2014)
- Jaime E. Castellanos, José I. Neissa, Sigrid J. Camacho, Dengue virus induces apoptosis in SH-SY5Y human neuroblastoma cells , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Claudia Viviana Barbosa, Carlos Enrique Muskus, Luz Yaneth Orozco, Adriana Pabón, Mutagenicity, genotoxicity and gene expression of Rad51C, Xiap, P53 and Nrf2 induced by antimalarial extracts of plants collected from the middle Vaupés region, Colombia , Biomedica: Vol. 37 No. 3 (2017)
- Benny Weiss-Steider, Yolanda Córdova, Itzen Aguiñiga-Sánchez, Edgar Ledesma-Martínez, Vanihamin Domínguez-Meléndez, Edelmiro Santiago-Osorio, Sodium caseinate and alfa-casein inhibit proliferation of the mouse myeloid cell line 32D clone 3 (32Dcl3) via TNF-α , Biomedica: Vol. 39 No. 2 (2019)
- Ricardo G. Díaz, Karina A. Salvatierra, Gustavo A. Silva, Enrique J. Deschutter, Fernando J. Bornay-Llinares, Lucrecia Acosta, First molecular characterization of Leishmania infantum species in patients infected with visceral leishmaniasis in Misiones province, Argentina , Biomedica: Vol. 39 No. 2 (2019)
- Karol Liseth Rueda-Concha , Ana Payares-Mercado , Jesús Guerra-Castillo , Jesús Melendrez , Yasmit Arroyo-Munive, Lily Martínez-Abad , Suljey Cochero , Eduar Elías Bejarano , Luis Enrique Paternina, Silent circulation of Leishmania infantum and Trypanosoma cruzi among urban dogs from Sincelejo city, Caribbean region of Colombia , Biomedica: Vol. 42 No. 4 (2022)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |