Biochemical characterization of Bothrops roedingeri Mertens, 1942 snake venom and its edematogenic, hemorrhagic, and myotoxic activities
Abstract
Introduction: Snakebite envenoming is considered by the World Health Organization (WHO) as a neglected tropical disease. Currently, Bothrops snake venoms are being studied intensively, but there is little knowledge about Bothrops roedingeri venom.
Objectives: To biochemically characterize B. roedingeri total venom and evaluate its myotoxic, edematogenic, and hemorrhagic activity.
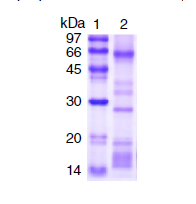
Materials and methods: We characterized B. roedingeri venom enzymatic activity by determining the phospholipase A2 and the proteolytic and fibrinogenolytic action using SDSPAGE electrophoresis while we characterized its venom toxicity by determining the minimum hemorrhagic dose, the minimum edema dose, and the local and systemic myotoxic effects.
Results: Bothrops roedingeri venom showed a PLA2 activity of 3.45 ± 0.11 nmoles/min, proteolytic activity of 0.145 ± 0.009 nmoles/min, and a fibrinogen coagulation index of 6.67 ± 1.33 seconds. On the other hand, it produced an minimum hemorrhagic dose of 24.5 μg, an minimum edema dose of 15.6 μg, and a pronounced local myotoxic effect evidenced by the elevation of plasma creatine kinase levels after intramuscular inoculation. The venom showed no systemic myotoxicity.
Conclusions: Bothrops roedingeri venom has local hemorrhagic, edematogenic, and myotoxic activity. Enzymatically, it has high PLA2 activity, which would be responsible for the myotoxic and edematogenic effects. It also has proteolytic activity, which could affect coagulation given its ability to degrade fibrinogen, and it causes bleeding through the metalloproteases.
Downloads
References
Chippaux JP. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins Incl Trop Dis. 2017;23:1-2. https://doi.org/10.1186/s40409-017-0127-6
Gutiérrez JM. Reducing the impact of snakebite envenoming in Latin America and the Caribbean: Achievements and challenges ahead. Trans R Soc Trop Med Hyg. 2014;108:530-7. https://doi.org/10.1093/trstmh/tru102
Ministerio de Salud. Número de casos de ofidismo según departamentos, Perú, 2014-2019. Fecha de consulta: 9 agosto del 2019. Disponible en: https://www.dge.gob.pe/portal/docs/vigilancia/sala/2019/SE46/ofidismo.pdf
Carrasco PA, Mattoni CI, Leynaud GC, Scrocchi GJ. Morphology, phylogeny and taxonomy of South American bothropoid pitvipers (Serpentes, Viperidae). Zool Scr. 2012;41:109-24. https://doi.org/10.1111/j.1463-6409.2011.00511.x
Navarrete ZM, Silva SW, Vargas ME. Las serpientes venenosas de importancia en la salud pública del Perú. Revista Electrónica de Veterinaria. 2010;XI:1-17.
Warrell DA. Snake bite. Lancet. 2010;375:77-88. https://doi.org/10.1016/S0140-6736(09)61754-2
Means R, Cabrera J, Moreno X, Amini R. Remote South American snakebite with extensive myonecrosis. Clin Pract Cases Emerg Med. 2017;25:47-9. https://doi.org/10.5811/cpcem.2016.11.31220
Huancahuire-Vega S, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Structural and functional characterization of brazilitoxins II and III (BbTX-II and -III), two myotoxins from the venom of Bothrops brazili snake. Toxicon. 2009;54:818-27. https://doi.org/10.1016/j.toxicon.2009.06.008
Guerra-Duarte C, Lopes-Peixoto J, Fonseca-de-Souza BR, Stransky S, Oliveira D, Schneider FS, et al. Partial in vitro analysis of toxic and antigenic activities of eleven Peruvian pitviper snake venoms. Toxicon. 2015;108:84-96. https://doi.org/10.1016/j.toxicon.2015.09.007
Tasoulis T, Isbister GK. A review and database of snake venom proteomes. Toxins (Basel). 2017;9:290:1-23. https://doi.org/10.3390/toxins9090290
Kohlhoff M, Borges MH, Yarleque A, Cabezas C, Richardson M, Sánchez EF. Exploring the proteomes of the venoms of the Peruvian pit vipers Bothrops atrox, B. barnetti and B. pictus. J Proteomics. 2012;75:2181-95. https://doi.org/10.1016/j.jprot.2012.01.020
Gomes Heleno MA, Baldasso PA, Ponce-Soto LA, Marangoni S. Biochemical characterization and pharmacological properties of new basic PLABrTX-I isolated from Bothrops roedingeri (Roedinger’s Lancehead) Mertens, 1942, snake venom. Biomed Res Int. 2013;591470:1-13. https://doi.org/10.1155/2013/591470
Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon. 1996;34:1149-55. https://doi.org/10.1016/0041-0101(96)00057-8
Erlanger BF, Kokowsky N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961;95:271-8. https://doi.org/10.1016/0003-9861(61)90145-x
Gallagher SR. One-dimensional SDS gel electrophoresis of proteins. Curr Protoc Protein Sci. 2012. https://doi.org/10.1002/0471140864.ps1001s68
Vilca-Quispe A, Ponce-Soto LA, Winck FV, Marangoni S. Isolation and characterization of a new serine protease with thrombin-like activity (TLBm) from the venom of the snake Bothrops marajoensis. Toxicon. 2010;55:745-53. https://doi.org/10.1016/j.toxicon.2009.11.006
Theakston RD, Reid HA. Development of simple standard assay procedures for the characterization of snake venom. Bull World Health Organ. 1983;61:949-56.
Damico DCS, Cruz Höfling MA, Cintra M, Leonardo MB, Calgarotto AK, Silva SL, et al. Pharmacological study of edema and myonecrosis in mice induced by venom of the bushmaster snake (Lachesis muta muta) and its basic Asp49 phospholipase A2 (LmTX-I). Protein J. 2008;27:384-91. https://doi.org/10.1007/s10930-008-9148-x
Gutiérrez JM, Ponce-Soto LA, Marangoni S, Lomonte B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: Comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon. 2008;51:80-92. https://doi.org/10.1016/j.toxicon.2007.08.007
Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Prim. 2017;3:17063:1-20. https://doi.org/10.1038/nrdp.2017.63
Huancahuire-Vega S, Ponce-Soto LA, Martins-De-Souza D, Marangoni S. Biochemical and pharmacological characterization of PhTX-I a new myotoxic phospholipase A 2 isolated from Porthidium hyoprora snake venom. Comp Biochem Physiol C Toxicol Pharmacol. 2011;154:108-19. https://doi.org/10.1016/j.cbpc.2011.03.013
Calgarotto AK, Damico DC, Ponce-Soto LA, Baldasso PA, Da Silva SL, Souza GH, et al. Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon. 2008;51:1509-19. https://doi.org/10.1016/j.toxicon.2008.03.030
Amorim FG, Menaldo DL, Carone SEI, Silva TA, Sartim MA, Pauw E De, et al. New insights on moojase, a thrombin-like serine protease from Bothrops moojeni snake venom. Toxins. 2018;10;1-19. https://doi.org/10.3390/toxins10120500
Escalante T, Rucavado A, Herrera C. Hemorrhage caused by snake venom metalloproteinases: A journey of discovery and understanding. Toxins. 2016,8,93:1-19. https://doi.org/10.3390/toxins8040093
Rojas E, Quesada L, Arce V, Lomonte B, Rojas G, Gutiérrez JM. Neutralization of four Peruvian Bothrops sp. snake venoms by polyvalent antivenoms produced in Perú and Costa Rica: Preclinical assessment. Acta Trop. 2005;93:85-95. https://doi.org/10.1016/j.actatropica.2004.09.008
Kayano AM, Simões-Silva R, Medeiros PSM, Maltarollo VG, Honorio KM, Oliveira E, et al. BbMP-1, a new metalloproteinase isolated from Bothrops brazili snake venom with in vitro antiplasmodial properties. Toxicon. 2015;106:30-41. https://doi.org/10.1016/j.toxicon.2015.09.005
García PJ, Yarlequé A, Bonilla-Ferreyra C, Pessah S, Vivas D, Sandoval GA, et al. Características bioquímicas y evaluación preclínica de un antiveneno botrópico liofilizado contra el veneno de la serpiente Bothrops atrox. Rev Peru Med Exp Salud Pública. 2008;25:386-90.
Markland JrFS, Swenson S. Snake venom metalloproteinases. Toxicon. 2013;62:3-18. https://doi.org/10.1016/j.toxicon.2012.09.004
Herrera C, Voisin M-B, Escalante T, Rucavado A, Nourshargh S, María Gutiérrez J, et al. Effects of PI and PIII snake venom haemorrhagic metalloproteinases on the microvasculature: A confocal microscopy study on the mouse cremaster muscle. PLoS ONE. 2016;11:168643:1-17. https://doi.org/10.1371/journal.pone.0168643
Teixeira C, Cury Y, Moreira V, Picolob G, Chaves F. Inflammation induced by Bothrops asper venom. Toxicon. 2009;54:988-97. https://doi.org/10.1016/j.toxicon.2009.03.019
Rengifo-Ríos AM, Muñoz-Gómez LM, Cabezas-Fajardo FA, Guerrero-Vargas JA. Edematic and coagulant effects caused by the venom of Bothrops rhombeatus neutralized by the ethanolic extract of Piper auritum. J Ethnopharmacol. 2019;242:112046:1-7. https://doi.org/10.1016/j.jep.2019.112046
Torres-Huaco DF, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Purification and characterization of a new weak hemorrhagic Metalloproteinase BmHF-1 from Bothrops marajoensis Snake Venom. Protein J. 2010; 29:407-16. https://doi.org/10.1007/s10930-010-9267-z
33. Huancahuire-Vega S, Ponce-Soto LA, Marangoni S. PhTX-II a basic myotoxic phospholipase A2 from Porthidium hyoprora snake venom, pharmacological characterization and amino acid sequence by mass spectrometry. Toxins (Basel). 2014;6:3077-97. https://doi.org/10.3390/toxins6113077
Gutiérrez JM, Escalante T, Hernández R, Gastaldello S, Saravia-Otten P, Rucavado A. Why is skeletal muscle regeneration impaired after myonecrosis induced by viperid snake venoms? Toxins. 2018;10:1-21. https://doi.org/10.3390/toxins10050182
Gutiérrez JM, Rucavado A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841-50. https://doi.org/10.1016/s0300-9084(00)01163-9
Some similar items:
- María José Sevilla-Sánchez, Diana Mora-Obando, Jhon Jairo Calderón , Jimmy Alexander Guerrero-Vargas, Santiago Ayerbe-González , Snakebite in the department of Nariño, Colombia: a retrospective analysis, 2008-2017 , Biomedica: Vol. 39 No. 4 (2019)
- María José Sevilla-Sánchez, Santiago Ayerbe-González, Eliana Bolaños-Bolaños, Snakebite biomedical and epidemiological aspects in the department of Cauca, Colombia, 2009-2018 , Biomedica: Vol. 41 No. 2 (2021)
- Viviana Alexandra Martínez-Villota , Paulo Francisco Mera-Martínez, José Darío Portillo-Miño, Massive acute ischemic stroke after Bothrops spp. envenomation in southwestern Colombia: Case report and literature review , Biomedica: Vol. 42 No. 1 (2022)
- Jairo Lizarazo , Ramón Patiño , Diego Lizarazo , Guadalupe Osorio, Fatal brain hemorrhage after Bothrops asper bite in the Catatumbo region of Colombia , Biomedica: Vol. 40 No. 4 (2020)
- Daniel Pineda, Kemel Ghotme, María Elvira Aldeco, Pablo Montoya, Snakebites in Yopal and Leticia, Colombia, 1996-1997 , Biomedica: Vol. 22 No. 1 (2002)
- Karen López, Lilian Catalina Tartaglino, Ingrid Iris Steinhorst, María Soledad Santini, Oscar Daniel Salomon, Risk factors, representations and practices associated with emerging urban human visceral leishmaniasis in Posadas, Argentina , Biomedica: Vol. 36 (2016): Suplemento 1, Microbiología médica
- Teddy Angarita-Sierra, Alejandro Montañez-Méndez , Tatiana Toro-Sánchez , Ariadna Rodríguez-Vargas , A case of envenomation by the false fer-de-lance snake Leptodeira annulata (Linnaeus, 1758) in the department of La Guajira, Colombia , Biomedica: Vol. 40 No. 1 (2020)
- Elba G. Rodríguez-Pérez, Alma Y. Arce-Mendoza, Roberto Saldívar-Palacios, Kevin Escandón-Vargas, Fatal Strongyloides stercoralis hyperinfection syndrome in an alcoholic diabetic patient from México , Biomedica: Vol. 40 No. Supl. 1 (2020): Mayo, Infecciones en el trópico
- Santiago Ayerbe-González , Gloria Esperanza Condiza-Benavides , María José Sevilla-Sánchez, First record of snakebites by Micrurus ortoni and Micrurus hemprichii (Serpentes: Elapidae) in Colombia and Perú , Biomedica: Vol. 41 No. 4 (2021)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























