Prognostic significance of telomerase reverse transcriptase promoter gen mutations in high grade meningiomas
Abstract
Introduction: Mutations in the promoter region of telomerase reverse transcriptase occur frequently in meningiomas.
Objective: To estimate the prognostic importance of telomerase reverse transcriptase mutations in Colombian patients with grades II and III meningioma.
Materials and methods: This was a multicenter retrospective cohort study of patients diagnosed with refractory or recurrent WHO grades II and III meningiomas, recruited between 2011 and 2018, and treated with systemic therapy (sunitinib, everolimus ± octreotide, and bevacizumab). Mutation status of the telomerase reverse transcriptase promoter was established by PCR.
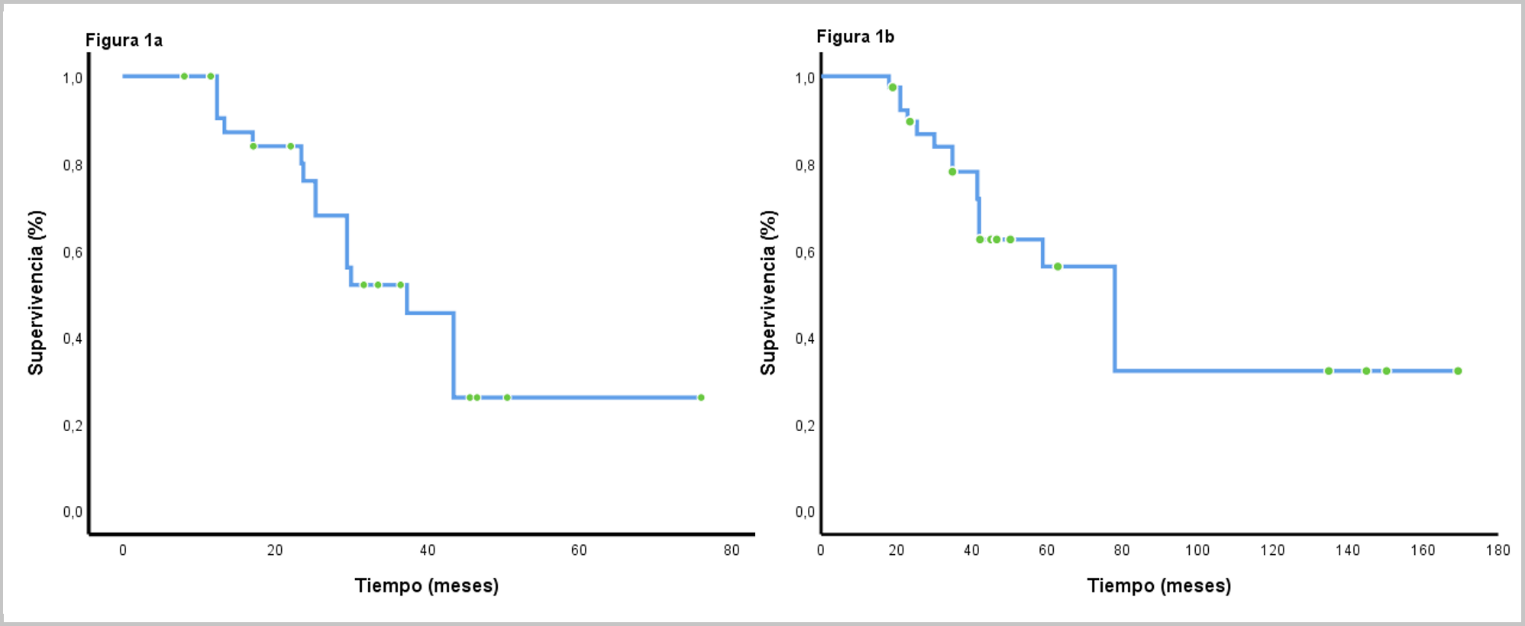
Results: Forty patients were included, of which telomerase reverse transcriptase mutations were found in 21 (52.5%), being C228T and C250T the most frequent variants with 87.5 % and 14.3 %, respectively. These were more frequent among patients with anaplastic meningiomas (p=0.18), with more than 2 recurrences (p=0.04); and in patients with parasagittal region and anterior fossa lesions (p=0.05). Subjects characterized as having punctual mutations were more frequently administered with everolimus, sunitinib and bevacizumab drug series (p=0.06). Overall survival was 23.7 months (CI95% 13.1-34.2) and 43.4 months (CI95% 37.5-49.3; p=0.0001) between subjects with and without mutations, respectively. Multivariate analysis showed that the number of recurrences and the presence of telomerase reverse transcriptase mutations were tthe only variables that negatively affected overall survival.
Conclusions: Mutations in telomerase reverse transcriptase allows the identification of high-risk patients and could be useful in the selection of the best medical treatment.
Downloads
References
Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: A clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-56. https://doi.org/10.1002/(sici)1097-0142(19990501)85:9<2046:aid-cncr23>3.0.co;2-m
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. https://doi.org/10.1007/s00401-007-0243-4
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl.5):v1-88. https://doi.org/10.1093/neuonc/nox158
Olar A, Wani KM, Sulman EP, Mansouri A, Zadeh G, Wilson CD, et al. Mitotic index is an independent predictor of recurrence-free survival in meningioma. Brain Pathol. 2015;25:266-75. https://doi.org/10.1111/bpa.12174
Wang YC, Chuang CC, Wei KC, Chang CN, Lee ST, Wu CT, et al. Long term surgical outcome and prognostic factors of atypical and malignant meningiomas. Sci Rep. 2016;6:35743. https://doi.org/10.1038/srep35743
Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, et al. WHO grade II and III meningiomas: A study of prognostic factors. J Neurooncol. 2009;95:367-75. https://doi.org/10.1007/s11060-009-9934-0
Agnihotri S, Suppiah S, Tonge PD, Jalali S, Danesh A, Bruce JP, et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nat Commun. 2017;8:186. https://doi.org/10.1038/s41467-017-00174-7
Christiaans I, Kenter SB, Brink HC, van Os TA, Baas F, van den Munckhof P, et al. Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet. 2011;48:93-7. https://doi.org/10.1136/jmg.2010.082420
Bi WL, Abedalthagafi M, Horowitz P, Agarwalla PK, Mei Y, Aizer AA, et al. Genomic landscape of intracranial meningiomas. J Neurosurg. 2016;125:525-35. https://doi.org/10.3171/2015.6.JNS15591
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077-80. https://doi.org/10.1126/science.1233009
Yuzawa S, Nishihara H, Tanaka S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016;33:237-47. https://doi.org 10.1007/s10014-016-0271-7
Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson WJ, Agarwalla PK, et al. Erratum: Genomic landscape of high-grade meningiomas. NPJ Genom Med. 2017;2:26. https://doi.org/10.1038/s41525-017-0023-6
Perry A, Banerjee R, Lohse CM, Kleinschmidt-DeMasters BK, Scheithauer BW. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12:183-90. https://doi.org/10.1111/j.1750-3639.2002.tb00433.x
Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. 2021;23:783-94. https://doi.org/10.1093/neuonc/noaa226
Shankar GM, Abedalthagafi M, Vaubel RA, Merrill PH, Nayyar N, Gill CM, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19:535-45. https://doi.org/10.1093/neuonc/now235
Smith MJ, O’Sullivan J, Bhaskar SS, Hadfield KD, Poke G, Caird J, et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45:295-8. https://doi.org/10.1038/ng.2552
Tauziede-Espariat A, Parfait B, Besnard A, Lacombe J, Pallud J, Tazi S, et al. Loss of SMARCE1 expression is a specific diagnostic marker of clear cell meningioma: A comprehensive immunophenotypical and molecular analysis. Brain Pathol. 2018;28:466-74. https://doi.org/10.1111/bpa.12524
Vasudevan H, Braunstein S, Phillips JJ, Pekmezci M, Wu A, Reis G, et al. GENE-04. Comprehensive genomic characterization of aggressive meningiomas identifies molecular signatures that predict clinical outcomes. Neuro-Oncology. 2017;19(Suppl.6):vi92-3. https://doi.org/10.1093/neuonc/nox168.379
Barthel FP, Wei W, Tang M, Martínez-Ledesma E, Hu X, Amin SB, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49:349-57. https://doi.org/10.1038/ng.3781
Yuan P, Cao J lin, Abuduwufuer A, Wang LM, Yuan XS, Lv W, et al. Clinical characteristics and prognostic significance of TERT promoter mutations in cancer: A cohort study and a meta-analysis. PLoS ONE. 2016;11:e0146803. https://doi.org/10.1371/journal.pone.0146803
Juratli TA, Thiede C, Koerner MVA, Tummala SS, Daubner D, Shankar GM, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8:109228-37. https://doi.org/10.18632/oncotarget.22650
Peyre M, Gauchotte G, Giry M, Froehlich S, Pallud J, Graillon T, et al. De novo and secondary anaplastic meningiomas: A study of clinical and histomolecular prognostic factors. Neuro Oncol. 2018;20:1113-21. https://doi.org/10.1093/neuonc/nox231
Sahm F, Schrimpf D, Olar A, Koelsche C, Reuss D, Bissel J, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108:djv377. https://doi.org/10.1093/jnci/djv377
Spiegl-Kreinecker S, Lötsch D, Neumayer K, Kastler L, Gojo J, Pirker C, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018;20:1584-93. https://doi.org/10.1093/neuonc/noy104
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803-20. https://doi.org/10.1007/s00401-016-1545-1
Huang RY, Bi WL, Weller M, Kaley T, Blakeley J, Dunn I, et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21:26-36. https://doi.org/10.1093/neuonc/noy137
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021-6. https://doi.org/10.1073/pnas.1303607110
Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. 2018;18:241-9. https://doi.org/10.1080/14737175.2018.1429920
Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24:184-9. https://doi.org/10.1111/bpa.12110
Mirian C, Duun-Henriksen AK, Juratli T, Sahm F, Spiegl-Kreinecker S, Peyre M, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: An individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91:378-87. https://doi.org/10.1136/jnnp-2019-322257
Harmancı AS, Youngblood MW, Clark VE, Coşkun S, Henegariu O, Duran D, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2018;9:16215. https://doi.org/10.1038/ncomms14433
Maier A, Brøchner CB, Bartek J, Eriksson F, Ugleholdt H, Broholm H, et al. Mitotic and proliferative indices in WHO grade III meningioma. Cancers (Basel). 2020;12:E3351. https://doi.org/10.3390/cancers12113351
Maier AD, Stenman A, Svahn F, Mirian C, Bartek J, Juhler M, et al. TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. 2021;31:61-9. https://doi.org/10.1111/bpa.12892
Williams EA, Santagata S, Wakimoto H, Shankar GM, Barker FG, Sharaf R, et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol Commun. 2020;8:171. https://doi.org/10.1186/s40478-020-01040-2
Barresi V, Simbolo M, Fioravanzo A, Piredda ML, Caffo M, Ghimenton C, et al. Molecular profiling of 22 primary atypical meningiomas shows the prognostic significance of 18q heterozygous loss and CDKN2A/B homozygous deletion on recurrence-free survival. Cancers (Basel). 2021;13:903. https://doi.org/10.3390/cancers13040903
Rutland JW, Gill CM, Loewenstern J, Arib H, Pain M, Umphlett M, et al. NF2 mutation status and tumor mutational burden correlate with immune cell infiltration in meningiomas. Cancer Immunol Immunother. 2021;70:169-76. https://doi.org/10.1007/s00262-020-02671-z
Gill CM, Loewenstern J, Rutland JW, Arib H, Pain M, Umphlett M, et al. SWI/SNF chromatin remodeling complex alterations in meningioma. J Cancer Res Clin Oncol. 2021;147:3431-40. https://doi.org/10.1007/s00262-020-02671-z
Olar A, Wani KM, Wilson CD, Zadeh G, DeMonte F, Jones DTW, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133:431-44. https://doi.org/10.1007/s00401-017-1678-x
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylationbased classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017;18:682-94. https://doi.org/10.1016/S1470-2045(17)3015
Nazem AA, Ruzevick J, Ferreira MJ. Advances in meningioma genomics, proteomics, and epigenetics: Insights into biomarker identification and targeted therapies. Oncotarget. 2020;11:4544-53. https://doi.org/10.18632/oncotarget.27841
Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, et al. Octreotide therapy in meningiomas: In vitro study, clinical correlation, and literature review. J Neurosurg. 2017;127:660-9. https://doi.org/10.3171/2016.8.JNS16995
Graillon T, Defilles C, Mohamed A, Lisbonis C, Germanetti AL, Chinot O, et al. Combined treatment by octreotide and everolimus: Octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J Neurooncol. 2015;124:33-43. https://doi.org/110.1007/s11060-015-1812-3
Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyrière H, et al. Everolimus and octreotide for patients with recurrent meningioma: Results from the Phase II CEVOREM Trial. Clin Cancer Res. 2020;26:552-7. https://doi.org/10.1158/1078-0432.CCR-19-2109
Cardona AF, Ruiz-Patiño A, Zatarain-Barrón ZL, Hakim F, Jiménez E, Mejía JA, et al. Systemic management of malignant meningiomas: A comparative survival and molecular marker analysis between Octreotide in combination with Everolimus and Sunitinib. PLoS ONE. 2019;14:e0217340. https://doi.org/10.1371/journal.pone.0217340
Hilton DA, Shivane A, Kirk L, Bassiri K, Enki DG, Hanemann CO. Activation of multiple growth factor signalling pathways is frequent in meningiomas. Neuropathology. 2016;36:250-61. https://doi.org/10.1111/neup.12266
Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17:116-21. https://doi.org/10.1093/neuonc/nou148
Scerrati A, Mongardi L, Visani J, Lofrese G, Cavallo MA, Fiorentino A, et al. The controversial role of Bevacizumab in the treatment of patients with intracranial meningioma: A comprehensive literature review. Expert Rev Anticancer Ther. 2020;20:197-203. https://doi.org/10.1080/14737140.2020.1736567
Franke AJ, Skelton WP, Woody LE, Bregy A, Shah AH, Vakharia K, et al. Role of bevacizumab for treatment-refractory meningiomas: A systematic analysis and literature review. Surg Neurol Int. 2018;9:133. https://doi.org/10.4103/sni.sni_264_17
Unterberger A, Nguyen T, Duong C, Kondajji A, Kulinich D, Yang I. Meta-analysis of adjuvant radiotherapy for intracranial atypical and malignant meningiomas. J Neurooncol. 2021;152:205-16. https://doi.org/10.1007/s11060-020-03674-7
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383-391. https://doi.org/10.1016/S1470-2045(16)30321-7
Stögbauer L, Stummer W, Senner V, Brokinkel B. Telomerase activity, TERT expression, hTERT promoter alterations, and alternative lengthening of the telomeres (ALT) in meningiomas - a systematic review. Neurosurg Rev. 2020;43:903-10. https://doi.org/10.1007/s10143-019-01087-3
Some similar items:
- Claudia Consuelo Rubiano, Moisés Wasserman, Identification of the gene sequence of telomerase catalytic subunit in Plasmodium falciparum. , Biomedica: Vol. 25 No. 1 (2005)
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























