Expression of ERG11, ERG3, MDR1 and CDR1 genes in Candida tropicalis
Abstract
Introduction. Drug resistance to azoles is a growing problem in the Candida genus.
Objective. To analyze molecularly the genes responsible for fluconazole resistance in Candida tropicalis strains.
Materials and methods. Nineteen strains, with and without exposure to fluconazole, were selected for this study. The expression of MDR1, CDR1, ERG11, and ERG3 genes was analyzed in sensitive, dose-dependent sensitive, and resistant strains exposed to different concentrations of the antifungal drug.
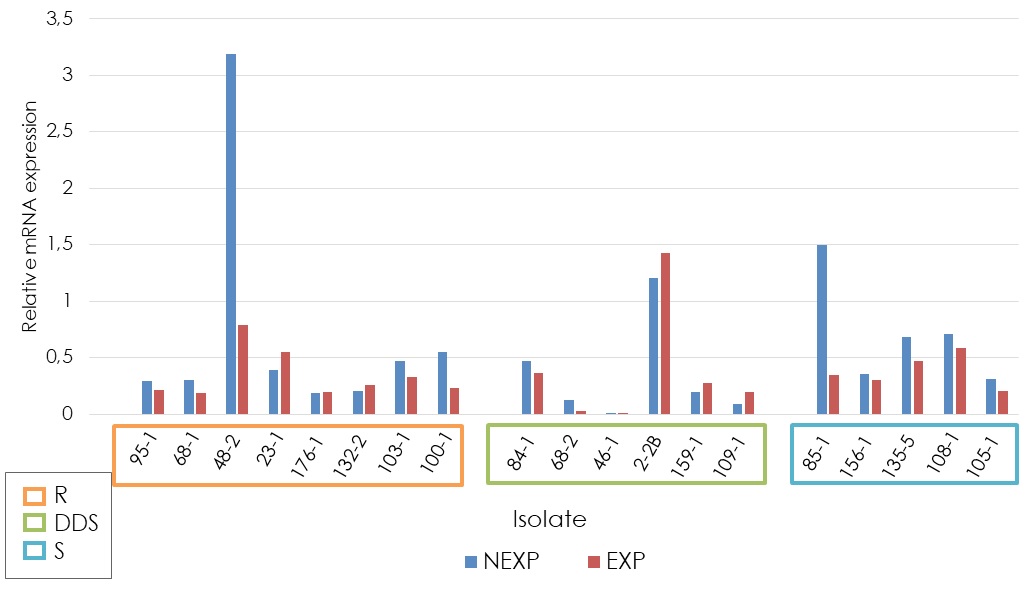
Results. MDR1, ERG11 and ERG3 genes were significantly overexpressed in the different sensitivity groups. CDR1 gene expression was not statistically significant among the studied groups. Seven of the eight fluconazole-resistant strains showed overexpression of one or more of the analyzed genes. In some dose-dependent sensitive strains, we found overexpression of CDR1, ERG11, and ERG3.
Conclusion. The frequency of overexpression of ERG11 and ERG3 genes indicates that they are related to resistance. However, the finding of dose-dependent resistant/sensitive strains without overexpression of these genes suggests that they are not exclusive to this phenomenon. More basic research is needed to study other potentially involved genes in the resistance mechanism to fluconazole.
Downloads
References
Bhattacharya S, Sae S, Fries BC. Candidiasis and mechanisms of antifungal resistance. Antibiotics (Basel). 2020;9:312. https://doi.org/10.3390/antibiotics9060312
Barac A, Cevik M, Colovic N, Lekovic D, Stevanovic G, Micic J, et al. Investigation of a healthcare-associated Candida tropicalis candidiasis cluster in a hematology unit and a systematic review of nosocomial outbreaks. Mycoses. 2020;63:326-33. https://doi.org/10.1111/myc.13048
Fan X, Xiao M, Liao K, Kudinha T, Wang H, Zhang L, et al. A notable increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (August 2009 to July 2014): Molecular epidemiology and clinical azole consumption. Front Microbiol. 2017;8:464. https://doi.org/10.3389/fmicb.2017.00464
Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86-96. https://doi.org/10.1016/j.bcp.2016.11.019
Arendrup MC, Patterson TF. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(Suppl.3):S445-51. https://doi.org/10.1093/infdis/jix131
Ksiezopolska E, Gabaldón T. Evolutionary emergence of drug resistance in Candida opportunistic pathogens. Genes (Basel). 2018;9:461. https://doi.org/10.3390/genes9090461
Sari S, Kart D, Öztürk N, Kaynak FB, Gencel M, Taşkor G, et al. Discovery of new azoles with potent activity against Candida spp. and Candida albicans biofilms through virtual screening. Eur J Med Chem. 2019;179:634-48. https://doi.org/10.1016/j.ejmech.2019.06.083
Cárdenas LY, Cárdenas JE. Mecanismos de resistencia a fluconazol expresados por Candida glabrata: una situación para considerar en la terapéutica. Inv Enf. 2020;20:112. https://doi.org/10.11144/Javeriana.ie22.mrfe
Tan TY, Hsu LY, Alejandria MM, Chaiwarith R, Chinniah T, Chayakulkeeree M, et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the AsiaPacific region. Med Mycol. 2016;54:471-7. https://doi.org/10.1093/mmy/myv114
Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 19972016. Open Forum Infect Dis. 2019;6(Suppl.1):S79-94. https://doi.org/10.1093/ofid/ofy358
Oliveira JS, Pereira VS, Castelo D, Cordeiro R, Sidrim JC, Brilhante SN, et al. The yeast, the antifungal, and the wardrobe: A journey into antifungal resistance mechanisms of Candida tropicalis. Can J Microbiol. 2020;66:377-88. https://doi.org/10.1139/cjm-2019-0531
Prasad R, Nair R, Banerjee A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet Biol. 2019;132:103252. https://doi.org/10.1016/j.fgb.2019.103252
Paul S, Singh S, Sharma D, Chakrabarti A, Rudramurthy SM, Ghosh AK. Dynamics of in vitro development of azole resistance in Candida tropicalis. J Glob Antimicrob Resist. 2020;22:553-61. https://doi.org/10.1016/j.jgar.2020.04.018
Fan X, Xiao M, Zhang D, Huang JJ, Wang H, Hou X, et al. Molecular mechanisms of azole resistance in Candida tropicalis isolates causing invasive candidiasis in China. Clin Microbiol Infect. 2019;25:885-91. https://doi.org/10.1016/j.cmi.2018.11.007
Hernández JS. Estudio básico-clínico de la colonización de especies de candida en adultos mayores al ingreso de cuidados intensivos. Manizales: Universidad de Caldas; 2015. p. 1-191.
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-22. https://doi.org/10.1373/clinchem.2008.112797
Rex J, Barbara D, Andes D, Arthintong B, Brown S, Chaturvedi V, et al. M27-A3 reference method for broth dilution antifungal susceptibility testing of yeast approved standard. Pennsylvania: Clinical and Laboratory Standards Institute; 2008. p. 1-13.
Jiang C, Dong D, Yu B, Cai G, Wang X, Ji Y, et al. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J Antimicrob Chemother. 2013;68:778-85. https://doi.org/10.1093/jac/dks481
Chen PY, Chuang YC, Wu UI, Sun HY, Wang JT, Sheng WH, et al. Mechanisms of azole resistance and trailing in Candida tropicalis bloodstream isolates. J Fungi. 2021;7:612. https://doi.org/10.3390/jof7080612
Choi MJ, Won EJ, Shin JH, Kim SH, Lee WG, Kim MN, et al. Resistance mechanisms and clinical features of fluconazole-nonsusceptible Candida tropicalis isolates compared with fluconazole-less-susceptible isolates. Antimicrob Agents Chemother. 2016;60:3653-61. https://doi.org/10.1128/AAC.02652-15
Sasani E, Yadegari MH, Khodavaisy S, Rezaie S, Salehi M, Getso MI. Virulence factors and azole-resistant mechanism of Candida tropicalis isolated from candidemia. Mycopathologia. 2021;186:847-56. https://doi.org/10.1007/s11046-021-00580-y
Rojas AE, Pérez JE, Hernández JS, Zapata Y. Quantitative analysis of the expression of fluconazole-resistant genes in strains of Candida albicans isolated from elderly people at their admission in an intensive care unit in Manizales, Colombia. Biomédica. 2020;40:153-65. https://doi.org/10.7705/biomedica.4723
Chapman B, Slavin M, Marriott D, Halliday C, Kidd S, Arthur I, et al. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother. 2017;72:1103-8. https://doi.org/10.1093/jac/dkw422
Vandeputte P, Larcher G, Bergès T, Renier G, Chabasse D, Bouchara JP. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob Agents Chemother. 2005;49:4608-15. https://doi.org/10.1128/AAC.49.11.4608-4615.2005
Jin L, Cao Z, Wang Q, Wang Y, Wang X, Chen H, et al. MDR1 overexpression combined with ERG11 mutations induce high-level fluconazole resistance in Candida tropicalis clinical isolates. BMC Infect Dis. 2018;18:162. https://doi.org/10.1186/s12879-018-3082-0
Pandey N, Tripathi M, Gupta MK, Tilak R. Overexpression of efflux pump transporter genes and mutations in ERG11 pave the way to fluconazole resistance in Candida tropicalis: A study from a North India region. J Glob Antimicrob Resist. 2020;22:374-8. https://doi.org/10.1016/j.jgar.2020.02.010
You L, Qian W, Yang Q, Mao L, Zhu L, Huang X, et al. ERG11 gene mutations and MDR1 upregulation confer pan-azole resistance in Candida tropicalis causing disseminated candidiasis in an acute lymphoblastic leukemia patient on posaconazole prophylaxis. Antimicrob Agents Chemother. 2017;61:e02496-16. https://doi.org/10.1128/AAC.02496-16
Barchiesi F, Calabrese D, Sanglard D, Falconi L, Caselli F, Giannini D, et al. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob Agents Chemother. 2000;44:1578-84. https://doi.org/10.1128/AAC.44.6.1578-1584.2000
Li QQ, Tsai HF, Mandal A, Walker BA, Noble JA, Fukuda Y, et al. Sterol uptake and sterol biosynthesis act coordinately to mediate antifungal resistance in Candida glabrata under azole and hypoxic stress. Mol Med Rep. 2018;17:6585-97. https://doi.org/10.3892/mmr.2018.8716
Lotfali E, Ghajari A, Kordbacheh P, Zaini F, Mirhendi H, Mohammadi R, et al. Regulation of ERG3, ERG6, and ERG11 genes in antifungal-resistant isolates of Candida parapsilosis. Iran Biomed J. 2017;21:275-81. https://doi.org/10.18869/acadpub.ibj.21.4.275
Eddouzi J, Parker JE, Vale-Silva LA, Coste A, Ischer F, Kelly S, et al. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob Agents Chemother. 2013;57:3182-93. https://doi.org/10.1128/AAC.00555-13
Jiang C, Dong D, Yu B, Cai G, Wang X, Ji Y, et al. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J Antimicrob Chemother. 2013;68:778-85. https://doi.org/10.1093/jac/dks481
Jiang C, Ni Q, Dong D, Zhang L, Li Z, Tian Y, et al. The role of UPC2 gene in azoleresistant Candida tropicalis. Mycopathologia. 2016;181:833-8. https://doi.org/10.1007/s11046-016-0050-3
Some similar items:
- Ana Elisa Rojas, Jorge Enrique Pérez, Johan Sebastián Hernández, Yuliana Zapata, Quantitative analysis of the expression of fluconazole-resistant genes in strains of Candida albicans isolated from elderly people at their admission in an intensive care unit in Manizales, Colombia , Biomedica: Vol. 40 No. 1 (2020)
- Catalina de Bedout, Julio Ayabaca, Ricardo Vega, Matilde Méndez, Axel R. Santiago, María Lucrecia Pabón, Angela Tabares, Myrtha Arango, Angela Restrepo, Vance Newell, Evaluation of Candida species' susceptibility to fluconazole with the disk diffusion method. , Biomedica: Vol. 23 No. 1 (2003)
- Isaura Torres, Juan E. Gallo , Oscar Mauricio Gómez , Álvaro Rúa-Giraldo , Juan G. McEwen , Ana María García , Gene expression profiles of ERG11, MDR1 and AFR1 in Cryptococcus neoformans var.grubbi from HIV patients , Biomedica: Vol. 42 No. 4 (2022)
- Kevin Ehemann, Andrés Contreras, Adriana Marcela Celis-Ramírez, In vitro sensitivity of Malassezia furfur isolates from HIV-positive and negative patients to antifungal agents , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- James Alexander Castillo , Natalia Afanasjeva, Method validation for the quantification of fluconazole and its organic impurities in raw material using high-performance liquid chromatography , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
Copyright (c) 2023 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























