In vitro and Quantitative and Structure Activity Relationship (QSAR) evaluation of the antifungal activity of terpenoid constituents of essential oils against Alternaria alternata and Fusarium oxysporum
Abstract
Introducción. Los géneros Alternaria y Fusarium contienen especies patógenas para los humanos y los cultivos. Para su control, se han utilizado diversos antifúngicos. Sin embargo, su uso desmedido ha contribuido al desarrollo de agentes patógenos resistentes. Una alternativa para buscar y desarrollar nuevos agentes antimicóticos son los aceites esenciales y sus componentes principales, los cuales poseen diversas actividades biológicas de interés para la medicina y en la preservación de alimentos.
Objetivo. Evaluar in vitro e in silico las actividades antifúngicas de terpenoides contra Alternaria alternata y Fusarium oxysporum.
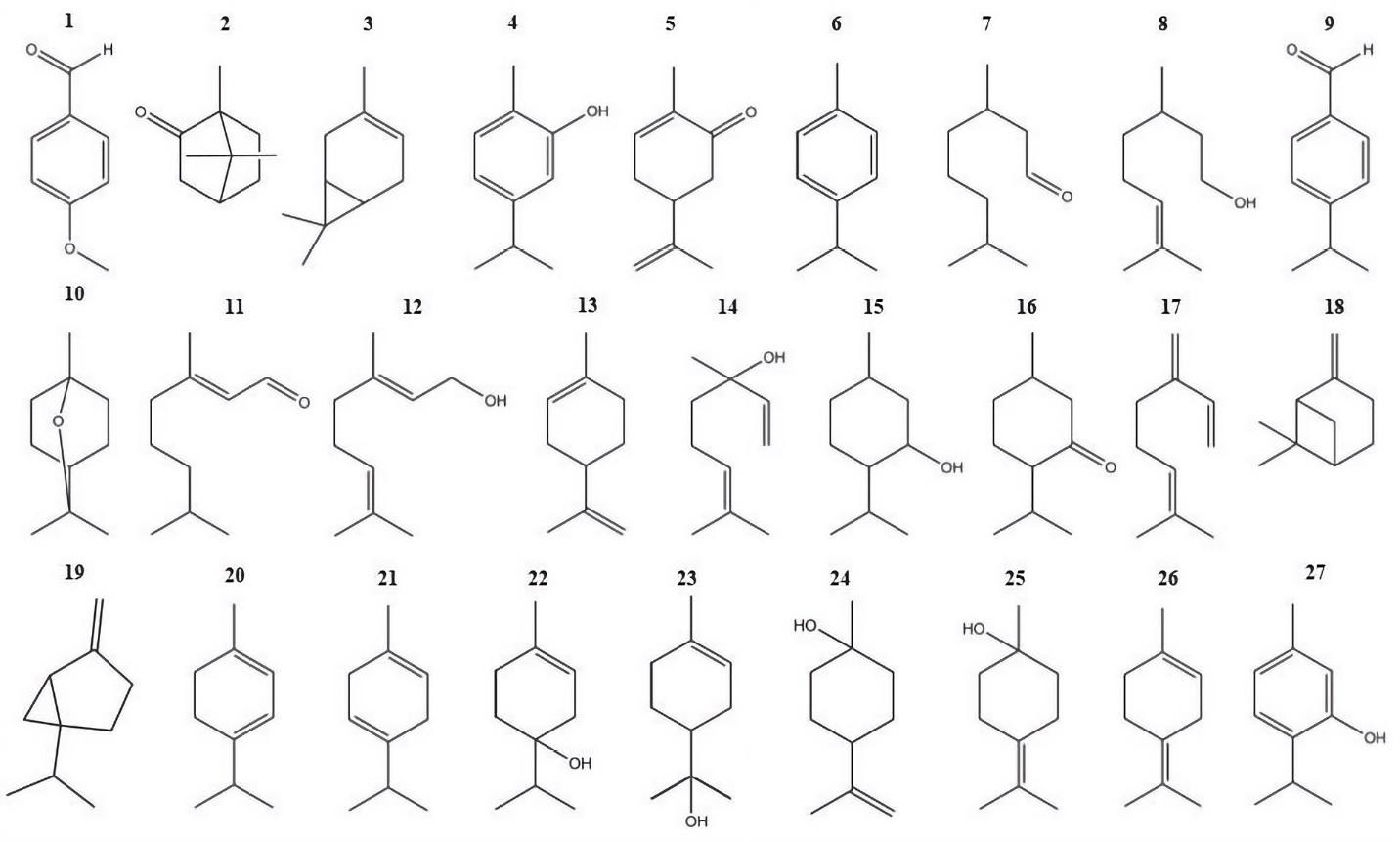
Materiales y métodos. Se evaluaron in vitro las concentraciones inhibitorias mínimas y las concentraciones fungicidas mínimas de 27 constituyentes de aceites esenciales
contra A. alternata y F. oxysporum. Además, mediante algoritmos genéticos, se crearon modelos cuantitativos de la relación estructura-actividad para determinar las propiedades estructurales y fisicoquímicas relacionadas con la actividad antifúngica.
Resultados. Los compuestos evaluados mostraron ser antifúngicos activos. El timol fue el compuesto con mayor actividad, con un valor de concentración inhibitoria mínima de 91.6 ± 28.8 μg/ml, tanto para Alternaria alternata como para Fusarium oxysporum. Los modelos cuantitativos de la relación estructura-actividad incluyeron la avidez por los lípidos y los fenoles como los principales grupos funcionales que contribuyen en la actividad antifúngica.
Conclusión. Los terpenoides poseen actividades antifúngicas relevantes para ser incorporados en el estudio de la química medicinal. La inclusión de pruebas in silico a la evaluación in vitro es una herramienta útil para la búsqueda y el diseño racional de derivados terpénicos como posibles agentes antifúngicos.
Downloads
References
Wolters PJ, Faino L, van Den Bosch TB, Evenhuis B, Visser RG, Seidl MF, et al. Gapless genome assembly of the potato and tomato early blight pathogen Alternaria solani. Mol Plant Microbe Interact. 2018;31:692-4. https://doi.org/10.1094/MPMI-12-17-0309-A
Edel-Hermann V, Lecomte C. Current status of Fusarium oxysporum Formae Speciales and races. Phytopathology. 2019;109:512-30. https://doi.org/10.1094/PHYTO-08-18-0320-RVW
Didehdar M, Khoshbayan A, Vesal S, Darban-Sarokhalil D, Razavi S, Chegini Z, et al. An overview of possible pathogenesis mechanisms of Alternaria alternata in chronic rhinosinusitis and nasal polyposis. Microb Pathog. 2021;155:104905. https://doi.org/10.1016/j.micpath.2021.104905
Velázquez-Contreras F, Acevedo-Parra H, Nuño-Donlucas SM, Núñez -Delicado E, Gabaldón JA. Development and characterization of a biodegradable PLA food packaging hold monoterpene–cyclodextrin complexes against Alternaria alternata. Polymers. 2019;11:1720. https://doi.org/10.3390/polym11101720
Dallé da Rosa P, Aquino V, Fuentefría AM, Goldani LZ. Diversity of Fusarium species causing invasive and disseminated infections. J Mycol Med. 2021;31:101137. https://doi.org/10.1016/j.mycmed.2021.101137
Chilaka CA, De Boevre M, Atanda OO, De Saeger S. The status of Fusarium mycotoxins in sub-Saharan Africa: A review of emerging trends and postharvest mitigation strategies towards food control. Toxins. 2017;9:19. https://doi.org/10.3390/toxins9010019
Tewoldemedhin YT, Mazzola M, Botha WJ, Spies CF, McLeod A. Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur J Plant Pathol. 2011;130:215-29. https://doi.org/10.1007/s10658-011-9747-9
Oli N, Singh UK, Jha SK. Antifungal activity of plant’s essential oils against post-harvest fungal disease of apple fruit. Forestry. Int J For Res Nepal. 2019;16:86-100. https://doi.org/10.3126/forestry.v16i0.28361
Ouda SM. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res J Microbiol. 2014;9:3442.
Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS. Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology. 2011;39:194-9. https://doi.org/10.5941/MYCO.2011.39.3.194
Miles TD, Miles LA, Fairchild KL, Wharton PS. Screening and characterization of resistance to succinate dehydrogenase inhibitors in Alternaria solani. Plant Pathology. 2014;63:155-64. https://doi.org/10.1111/ppa.12077
Lucas JA, Hawkins NJ, Fraaije BA. The evolution of fungicide resistance. Adv Appl Microbiol. 2015;90:29-92. https://doi.org/10.1016/bs.aambs.2014.09.001
Neoh CF, Leung L, Vajpayee RB, Stewart K, Kong DC. Treatment of Alternaria keratitis with intrastromal and topical caspofungin in combination with intrastromal, topical, and oral voriconazole. Ann Pharmacother. 2011;45:e24. https://doi.org/10.1345/aph.1P586
Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, de Hoog GS, Meis JF. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother. 2014;70:1068-71. https://doi.org/10.1093/jac/dku505
Al-Hatmi AM, Meis JF, de Hoog GS. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathog. 2016;12:e1005464. https://doi.org/10.1371/journal.ppat.1005464
Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86. https://doi.org/10.3390/ph10040086
Debonne E, van Bockstaele F, De Leyn I, Devlieghere F, Eeckhout M. Validation of in-vitro antifungal activity of thyme essential oil on Aspergillus niger and Penicillium paneum through application in par-baked wheat and sourdough bread. LWT Food Sci Technol. 2018;87:368-78. https://doi.org/10.1016/j.lwt.2017.09.007
Mandras N, Nostro A, Roana J, Scalas D, Banche G, Ghisetti V, et al. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement Med Ther. 2016;16:330. https://doi.org/10.1186/s12906-016-1316-5
Mokbel AA, Alharbi AA. Antifungal effects of basil and camphor essential oils against Aspergillus flavus and A. parasiticus. Aust J Crop Sci. 2015;9:532. https://doi.org/10.3316/INFORMIT.298113037575673
Bahraminejad S, Seifolahpour B, Amiri R. Antifungal effects of some medicinal and aromatic plant essential oils against Alternaria solani. J Crop Prot. 2017;5:603-16.
Roselló J, Sempere F, Sanz-Berzosa I, Chiralt A, Santamarina MP. Antifungal activity and potential use of essential oils against Fusarium culmorum and Fusarium verticillioides. J Essent Oil-Bear Plants. 2015;18:359-67. https://doi.org/10.1080/0972060X.2015.1010601
Sharma A, Rajendran S, Srivastava A, Sharma S, Kundu B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. Lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J Biosci Bioeng. 2017;123:308-13. https://doi.org/10.1016/j.jbiosc.2016.09.011
Cavaleiro C, Salgueiro L, Gonçalves MJ, Hrimpeng K, Pinto J, Pinto E. Antifungal activity of the essential oil of Angelica major against Candida, Cryptococcus, Aspergillus and dermatophyte species. J Nat Med. 2015;69:241-8. https://doi.org/10.1007/s11418-014-0884-2
Basak S, Guha P. Use of predictive model to describe sporicidal and cell viability efficacy of betel leaf (Piper betle L.) essential oil on Aspergillus flavus and Penicillium expansum and its antifungal activity in raw apple juice. LWT Food Sci Technol. 2017;80:510-6. https://doi.org/10.1016/j.lwt.2017.03.024
Pinto E, Gonçalves MJ, Cavaleiro C, Salgueiro L. Antifungal activity of Thapsia villosa essential oil against Candida, Cryptococcus, Malassezia, Aspergillus and Dermatophyte species. Molecules. 2017;22:1595. https://doi.org/10.3390/molecules22101595
Rao J, Chen B, McClements DJ. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu Rev Food Sci Technol. 2019;10:365-87. https://doi.org/10.1146/annurev-food-032818-121727
Surabhi S, Singh BK. Computer aided drug design: An overview. J Drug Deliv Ther. 2018;8:504-9. https://doi.org/10.22270/jddt.v8i5.1894
Medina-Franco JL. Grand challenges of computer-aided drug design: The road ahead. Front Drug Des Discov. 2021;1:728551. https://doi.org/10.3389/fddsv.2021.728551
Toropov AA, Toropova AP. QSPR/QSAR: State-of-art, weirdness, the future. Molecules. 2020;25:1292. https://doi.org/10.3390/molecules25061292
Achary PG. Applications of quantitative structure–activity relationships (QSAR) based virtual screening in drug design: A review. Mini-Rev Med Chem. 2020;20:1375-88. https://doi.org/10.2174/1389557520666200429102334
Rasooli I, Mirmostafa SA. Bacterial susceptibility to and chemical composition of essential oils from Thymus kotschyanus and Thymus persicus. J Agric Food Chem. 2003;51:2200-5. https://doi.org/10.1021/jf0261755
Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB. Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol. 2008;122:135-9. https://doi.org/10.1016/j.ijfoodmicro.2007.11.048
Andrade-Ochoa S, Correa-Basurto J, Rodríguez-Valdez LM, SánchezTorres LE, Nogueda-Torres B, Nevárez-Moorillón GV. In vitro and in silico studies of terpenes, terpenoids and related compounds with larvicidal and pupaecidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Chem Cent J. 2018;12:53.
https://doi.org/10.1186/s13065-018-0425-2
Andrade-Ochoa S, García-Machorro J, Bello M, Rodríguez-Valdez LM, Flores-Sandoval CA, Correa-Basurto J. QSAR, DFT and molecular modeling studies of peptides from HIV-1 to describe their recognition properties by MHC-I. J Biomol Struct Dyn. 2018;36:2312-30. https://doi.org/10.1080/07391102.2017.1352538
Todeschini R. (2006). Molecular descriptors, QSAR, chemometrics and chemoinformatics – Dragon software for Windows (Version 5.4) [Windows]. Milano: Talete SRL. Available at: http://www.talete.mi.it/
Koopmans T. Über die zuordnungwellenfunktionen von und zuden eigenwerteneinzelnen elektronenátomoseines. Physica. 1934;1:104-13.
Miertus S, Scrocco E, Tomasi J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem Phys. 1981;55:117-29. https://doi.org/10.1016/0301-0104(81)85090-2
Todeschini R, Consonni V, Mauri A, Pavan M. MobyDigs software for regression and classification models by genetic algorithms. Data Handling Sci Technol. 2003;23:141-67. https://doi.org/10.1016/S0922-3487(03)23005-7
García-Ruiz JC, Amutio E, Pontón J. Infección fúngica invasora en pacientes inmunodeficientes. Rev Iberoam Micol. 2004;21:55-62.
Holding KJ, Dworkin MS, Wan PCT, Hanson DL, Klevens RM, Jones JL, et al. Adult and adolescent spectrum of HIV disease project. Aspergillosis among people infected with human immunodeficiency virus: Incidence and survival. Clin Infect Dis. 2000;31:1253-7. https://doi.org/10.1086/317452
Fraeyman S, Croubels S, Devreese M, Antonissen G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins. 2017;9:228. https://doi.org/10.3390/toxins9070228
Gnat S, Łagowski D, Nowakiewicz A, Dyląg M. A global view on fungal infections in humans and animals: Opportunistic infections and microsporidioses. J Appl Microbiol. 2021;131:2095-113. https://doi.org/10.1111/jam.15032
Bassolé IHN, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989-4006. https://doi.org/10.1016/j.foodchem.2016.09.179
Hu Y, Zhang J, Kong W, Zhao G, Yang M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017;220:1-8. https://doi.org/10.1016/j.foodchem.2016.09.179
Batista BG, Chaves MAD, Reginatto P, Saraiva OJ, Fuentefría AM. Human fusariosis: An emerging infection that is difficult to treat. Rev Soc Bras Med Trop. 2020;53. https://doi.org/10.1590/0037-8682-0013-2020
Portillo-Ruiz MC, Viramontes-Ramos S, Muñoz-Castellanos LN, Gastelum-Franco MG, Nevárez-Moorillón GV. Antifungal activity of Mexican oregano (Lippia berlandieri Shauer). J Food Prot. 2005;68:2713-7. https://doi.org/10.4315/0362-028X-68.12.2713
Ávila-Sosa R, Gastélum-Franco MG, Camacho-Dávila A, Torres-Muñoz JV, Nevárez-Moorillón GV. Extracts of Mexican oregano (Lippia berlandieri Schauer) with antioxidant and antimicrobial activity. Food Bioproc Tech. 2010;3:434-40. https://doi.org/10.1007/s11947-008-0085-7
Portillo-Ruiz MC, Sánchez RAS, Ramos SV, Muñoz JVT, NevárezMoorillón GV. Antifungal effect of Mexican oregano (Lippia berlandieri Schauer) essential oil on a wheat flour-based Medium. J Food Sci. 2012;77. https://doi.org/10.1111/j.1750-3841.2012.02821.x
Andrade-Ochoa S, Chacón-Vargas KF, Sánchez-Torres LE, RiveraChavira BE, Nogueda-Torres B, Nevárez-Moorillón GV. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes. 2021;11:405. https://doi.org/10.3390/membranes11060405
Hadizadeh I, Peivastegan B, Hamzehzarghani H. Antifungal activity of essential oils from some medicinal plants of Iran against Alternaria alternata. Am J Appl Sci. 2009;6:857-61. https://doi.org/10.3844/ajassp.2009.857.861
Perina FJ, Amaral DC, Fernandes RS, Labory CR, Teixeira GA, Alves E. Thymus vulgaris essential oil and thymol against Alternaria alternata (Fr.) Keissler: Effects on growth, viability, early infection and cellular mode of action. Pest Manag Sci. 2015;71:1371-8. https://doi.org/10.1002/ps.3933
Liu Y, Liu S, Luo X, Wu X, Ren J, Huang X, et al. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol Technol. 2022;192:112025. https://doi.org/10.1016/j.postharvbio.2022.112025
Gao T, Zhou H, Zhou W, Hu L, Chen J, Shi Z. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules. 2016;21:770. https://doi.org/10.3390/molecules21060770
Numpaque MA, Oviedo LA, Gil JH, García CM, Durango DL. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop Plant Pathol. 2011;36:313. https://doi.org/10.1590/S1982-56762011000100001
Moghtader M. Antifungal effects of the essential oil from Thymus vulgaris L. and comparison with synthetic thymol on Aspergillus niger. J Yeast Fungal Res. 2012;3:83-8. https://doi.org/0.5897/JYFR12.023
Zhang J, Ma S, Du S, Chen S, Sun H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J Food Sci Technol. 2019;56:2611-20. https://doi.org/10.1007/s13197-019-03747-0
Chen Y, Zeng H, Tian J, Ban X, Ma B, Wang Y. Antifungal mechanism of essential oil from Anethum graveolens seeds against Candida albicans. J Med Microbiol. 2013;62:1175-83. https://doi.org/10.1099/jmm.0.055467-0
Ahmad A, Khan A, Kumar P, Bhatt RP, Manzoor N. Antifungal activity of Coriaria nepalensis essential oil by disrupting ergosterol biosynthesis and membrane integrity against Candida. Yeast. 2011;28:611-7. https://doi.org/10.1002/yea.1890
Shen Q, Zhou W, Li H, Hu L, Mo H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the induction of nitric oxide. PLoS ONE. 2016;11:e0155647. https://doi.org/10.1371/journal.pone.0155647
Haque E, Irfan S, Kamil M, Sheikh S, Hasan A, Ahmad A, et al. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology. 2016;85:436-43. https://doi.org/10.1134/S0026261716040093
Hao Y, Zhang J, Sun C, Chen X, Wang Y, Lu H, et al. Thymol induces cell death of Fusarium oxysporum f. sp. niveum via triggering superoxide radical accumulation and oxidative injury in vitro. Agronomy. 2023;13:189. https://doi.org/10.3390/agronomy13010189
Some similar items:
- Valeria Velásquez-Zapata, Katherine Palacio-Rúa, Luz E. Cano, Adelaida Gaviria-Rivera, Assessment of genotyping markers in the molecular characterization of a population of clinical isolates of Fusarium in Colombia , Biomedica: Vol. 42 No. 1 (2022)
- Sonia Isabel Cuervo-Maldonado, José Camilo Álvarez-Rodríguez, Cristian Leonardo Cubides, Juan Camilo Barrera, Juan Diego Montañez-Abril, Erika Paola Vergara-Vela, Carlos Humberto Saavedra-Trujillo, María José López-Mora, Gloria Elena Mora-Figueroa, Adriana Celis-Ramírez , Rose Mary Jaramillo-Calle, Rafael Parra-Medina, Fusariosis in cancer patients: 13 case series report and literature review , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
Copyright (c) 2023 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























