Production of recombinant proteins from Plasmodium falciparum in Escherichia coli
Abstract
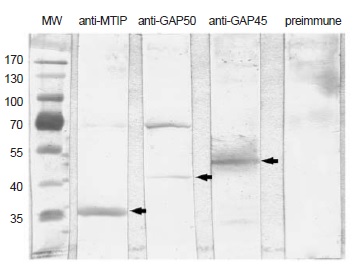
Introduction: The production of recombinant proteins is essential for the characterization and functional study of proteins from Plasmodium falciparum. However, the proteins of P. falciparum are among the most challenging to express, and when expression is achieved, the recombinant proteins usually fold incorrectly and lead to the formation of inclusion bodies. Objective: To obtain and purify four recombinant proteins and to use them as antigens to produce polyclonal antibodies. The production efficiency and solubility were evaluated as the proteins were expressed in two genetically modified strains of Escherichia coli to favor the production of heterologous proteins (BL21-CodonPlus (DE3)-RIL and BL21-pG-KJE8). Materials and methods: The four recombinant P. falciparum proteins corresponding to partial sequences of PfMyoA (Myosin A) and PfGAP50 (gliding associated protein 50), and the complete sequences of PfMTIP (myosin tail interacting protein) and PfGAP45 (gliding associated protein 45), were produced as glutathione S-transferase-fusion proteins, purified and used for immunizing mice. Results: The protein expression was much more efficient in BL21-CodonPlus, the strain that contains tRNAs that are rare in wild-type E. coli, compared to the expression in BL21-pG-KJE8. In spite of the fact that BL21-pG-KJE8 overexpresses chaperones, this strain did not minimize the formation of inclusion bodies. Conclusion: The use of genetically modified strains of E. coli was essential to achieve high expression levels of the four evaluated P. falciparum proteins and lead to improved solubility of two of them. The approach used here allowed us to obtain and purify four P. falciparum proteins in enough quantity to produce polyclonal antibodies in mice, and a fair amount of two pure and soluble recombinant proteins for future assays.
Downloads
References
World Health Organization. World Malaria Report 2014: Summary. Accessed: April 1st, 2015. Available from: http://apps.who.int/iris/bitstream/10665/160458/1/WHO_HTM_GMP_2015.2_eng.pdf?ua=1.
Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755-66. http://dx.doi.org/10.1016/j.cell.2006.02.006.
Frénal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343-57. http://dx.doi.org/10.1016/j.chom.2010.09.002.
Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197-208. http://dx.doi.org/10.1074/jbc.M509807200.
Overton TW. Recombinant protein production in bacterial hosts. Drug Discov Today. 2014;19:590-601. http://dx.doi.org/10.1016/j.drudis.2013.11.008.
Horrocks P, Bowman S, Kyes S, Waters AP, Craig A. Entering the post-genomic era of malaria research. Bull WHO. 2000;78:1424-37.
Yadava A, Ockenhouse CF. Effect of codon optimisation on expression levels of a functionally folded malaria vaccine candidate in prokaryotic and eukaryotic expression system. Infect Immun. 2003;71:4961-9. http://dx.doi.org/10.1128/IAI.71.9.4961-4969.2003.
Flick K, Ahuja S, Chene A, Bejarano MT, Chen Q. Optimized expression of Plasmodium falciparum erythrocyte membrane protein 1 domains in Escherichia coli. Malar J. 2004;3:50. http://dx.doi.org/10.1186/1475-2875-3-50.
Villaverde A, Carrio MM. Protein aggregation in recombinant bacteria: Biological role of inclusion bodies. Biotechnol Lett. 2003;25:1385-95. http://dx.doi.org/10.1023/A:1025024104862.
Wasserman M, Contreras J, Pinilla G, Rojas MO, Páez A, Caminos E. Plasmodium falciparum: Characterization of a 0.7-kbp, moderately repetitive sequence. Exp Parasitol. 1995;81:165-71. http://dx.doi.org/10.1006/expr.1995.1105.
Sørensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact. 2005;4:1. http://dx.doi.org/10.1186/1475-2859-4-1.
Scheer JM, Ryan CA. A method for the quantitative recovery of proteins from polyacrylamide gels. Anal Biochem. 2001; 298:130-2. http://dx.doi.org/10.1006/abio.2001.5384.
Harlow E, Lane D. Antibodies: A laboratory manual. First edition. New York: Cold Spring Harbor Laboratory Press; 1988. p. 67, 92-120. http://dx.doi.org/10.1002/jobm.3620300304.
Jonasson P, Liljeqvist S, Nygren PA, Ståhl S. Genetic design for facilitated production and recovery of recombinant proteins in Escherichia coli. Biotechnol Appl Biochem. 2002;35:91-105. http://dx.doi.org/10.1042/BA20010099.
Donovan RS, Robinson CW, Glick BR. Optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol. 1996;16:145-54. http://dx.doi.org/10.1007/BF01569997.
Mehlin C, Boni E, Buckner FS, Engel L, Feist T, Gelb MH, et al. Heterologous expression of proteins from Plasmodium falciparum: Results from 1000 genes. Mol Biochem Parasitol. 2006;148:144-60. http://dx.doi.org/10.1016/j.molbiopara.2006.03.011.
Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, et al. Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol Biochem Parasitol. 2007;151:100-10. http://dx.doi.org/10.1016/j.molbiopara.2006.10.011.
Armstrong DJ, Roman A. The anomalous electrophoretic behavior of the human papillomavirus type 16 E7 protein is due to the high content of acidic amino acid residues. Biochem Biophys Res Commun. 1993;192:1380-7. http://dx.doi.org/10.1006/bbrc.1993.1569.
Iakoucheva LM, Kimzey AL, Masselon CD, Smith RD, Dunker AK, Ackerman EJ. Aberrant mobility phenomena of the DNA repair protein XPA. Protein Sci. 2001;10:1353-62. http://dx.doi.org/10.1110/ps.ps.40101.
Shi W, Huang Y, Sutton-Smith M, Tissot B, Panico M, Morris HR, et al. A filovirus-unique region of Ebola virus nucleoprotein confers aberrant migration and mediates its incorporation into virions. J Virol. 2008;82:6190-9. http://dx.doi.org/10.1128/JVI.02731-07.
Jones ML, Kitson EL, Rayner JC. Plasmodium falciparum erythrocyte invasion: A conserved myosin associated complex. Mol Biochem Parasitol. 2006;147:74-84. http://dx.doi.org/10.1016/j.molbiopara.2006.01.009.
Rees-Channer RR, Martin SR, Green JL, Bowyer PW, Grainger M, Molloy JE, et al. Dual acylation of the 45 kDa gliding-associated protein (GAP45) in Plasmodium falciparum merozoites. Mol Biochem Parasitol. 2006;149:113-6. http://dx.doi.org/10.1016/j.molbiopara.2006.04.008.
Baca AM, Hol WG. Overcoming codon bias: A method for high-level overexpression of Plasmodium and other AT-rich parasite genes in Escherichia coli. Int J Parasitol. 2000;30:113-8. http://dx.doi.org/10.1016/S0020-7519(00)00019-9.
Karmodiya K, Srivastav RK, Surolia N. Production and purification of refolded recombinant Plasmodium falciparum beta-ketoacyl-ACP reductase from inclusion bodies. Protein Expr Purif. 2005;42:131-6. http://dx.doi.org/10.1016/j.pep.2005.02.008.
Carstens P. Use of tRNA-supplemented host strains for expression of heterologous genes in E. coli. In: Vaillancourt PE, editor. E. coli gene expression protocols. Totowa, NJ: Humana Press; 2003. p. 225-33. http://dx.doi.org/10.1385/1-59259-301-1:225.
Goldman E, Rosenberg AH, Zubay G, Studier FW. Consecutive low-usage leucine codons block translation only when near the 5' end of a message in Escherichia coli. J Mol Biol. 1995;245:467-73. http://dx.doi.org/10.1006/jmbi.1994.0038.
Chen GF, Inouye M. Suppression of the negative effect of minor arginine codons on gene expression; preferential usage of minor codons within the first 25 codons of the Escherichia coli genes. Nucleic Acids Res. 1990;18:1465-73. http://dx.doi.org/10.1093/nar/18.6.1465.
Rosano GL, Ceccarelli EA. Rare codon content affects the solubility of recombinant proteins in a codon bias-adjusted Escherichia coli strain. Microb Cell Fact. 2009;8:41. http://dx.doi.org/10.1186/1475-2859-8-41.
Schumann W, Ferreira LC. Production of recombinant proteins in Escherichia coli. Genet Mol Biol. 2004;27:442-53. http://dx.doi.org/10.1590/S1415-47572004000300022.
Barth S, Huhn M, Matthey B, Klimka A, Galinski EA, Engert A. Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl Environ Microbiol. 2000;66:1572-9. http://dx.doi.org/10.1128/AEM.66.4.1572-1579.2000.
Schäffner J, Winter J, Rudolph R, Schwarz E. Cosecretion of chaperones and low-molecular-size medium additives increases the yield of recombinant disulfide-bridged proteins. Appl Environ Microbiol. 2001;67:3994-4000. http://dx.doi.org/10.1128/AEM.67.9.3994-4000.2001.
Choi JH, Lee SY. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol. 2004;64:625-35. http://dx.doi.org/10.1007/s00253-004-1559-9.
Wall JG, Plückthun A. Effects of overexpressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:507-16. http://dx.doi.org/10.1016/0958-1669(95)80084-0.
Martínez-Alonso M, García-Fruitós E, Ferrer-Miralles N, Rinas U, Villaverde A. Side effects of chaperone gene co-expression in recombinant protein production. Microb Cell Fact. 2010;9:64. http://dx.doi.org/10.1186/1475-2859-9-64.
Some similar items:
- Amanda Maestre, Jaime Carmona-Fonseca, Amanda Maestre, Alta frecuencia de mutaciones puntuales en pfcrt de Plasmodium falciparum y emergencia de nuevos haplotipos mutantes en Colombia , Biomedica: Vol. 28 No. 4 (2008)
- Silvia Blair, Ana Mercedes Rada, Carolina Moreno, Successful in vitro culture of Plasmodium falciparum gametocytes , Biomedica: Vol. 28 No. 4 (2008)
- Jaime Carmona-Fonseca, Eliana Arango, Silvia Blair, Gametocytemia in falciparum malaria treated with amodiaquine or artesunate , Biomedica: Vol. 28 No. 2 (2008)
- Silvia Blair, Eliana Arango, Jaime Carmona Fonseca, In vitro susceptibility of Colombian Plasmodium falciparum isolates to different antimalarial drugs , Biomedica: Vol. 28 No. 2 (2008)
- John Alexander Galindo, Fabio Aníbal Cristiano, Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Point mutations in dihydrofolate reductase and dihydropteroate synthase genes of Plasmodium falciparum from three endemic malaria regions in Colombia , Biomedica: Vol. 30 No. 1 (2010)
- Nohora Marcela Mendoza, Marisol García, Liliana Jazmín Cortés, Claudia Vela, Rigoberto Erazo, Pilar Pérez, Olga Lucía Ospina, Javier Darío Burgos, Evaluation of two rapid diagnostic tests, NOW® ICT Malaria Pf/Pv and OptiMAL®, for diagnosis of malaria , Biomedica: Vol. 27 No. 4 (2007)
- Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Ricardo Sánchez, Clinical profiles of patients with uncomplicated Plasmodum falciparum malaria in northwestern Colombia , Biomedica: Vol. 27 No. 4 (2007)
- Adriana Pabón, Gonzalo Álvarez, Jorge Yánez, Carlos Céspedes, Yensa Rodríguez, Ángela Restrepo, Silvia Blair, Evaluation of ICT malaria immunochromatographic Binax NOW® ICT P.f/P.v test for rapid diagnosis of malaria in a Colombian endemic area , Biomedica: Vol. 27 No. 2 (2007)
- Nadia Yadira Castañeda, Jacqueline Chaparro-Olaya, Jaime E. Castellanos, Production and characterization of a polyclonal antibody against rabies virus phosphoprotein , Biomedica: Vol. 27 No. 2 (2007)
- Sandra Milena Barrera, Manuel Alberto Pérez, Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Genotypic survery of Plasmodium falciparum based on the msp1, msp2 and glurp genes by multiplex PCR , Biomedica: Vol. 30 No. 4 (2010)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























