Partial inhibition of two genes that encode spliceosomal proteins in Giardia intestinalis
Abstract
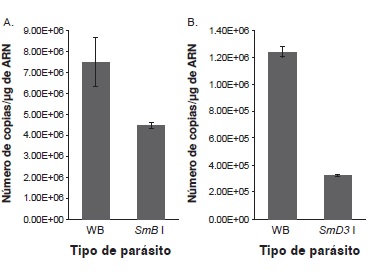
Introduction. Giardia intestinalis is an early divergent organism that was recently shown to have introns. The machinery responsible for the removal of introns in higher eukaryotes is the spliceosome, which consists of five ribonucleoproteins. Each of these ribonucleoproteins has a small nuclear RNA, a set of seven Sm proteins (B, D1, D2, D3, E, F and G) and several specific proteins. Some genes that encode spliceosome proteins have been bioinformatically identified in the parasite genome. Although it is assumed that the spliceosome is responsible for splicing in this parasite, biochemical characterization is lacking. Objective. To inhibit two G. intestinalis spliceosome protein genes in order to determine whether this inhibition affects parasite growth or encystation. Materials and methods. Antisense sequences of the genes encoding the spliceosomal parasite proteins SmB and SmD3 were cloned into a specific G. intestinalis vector. G. intestinalis individuals were subsequently transfected with the recombinant vectors and those parasites that incorporated the vector were selected. A decrease in mRNA levels by real-time PCR was confirmed and the growth and encystation in wild and transfected parasites was assessed. Results. A decrease of 40% and 70% of SmB and SmD3 mRNA levels, respectively, was observed. Growth and encystation in these parasites were not affected. Conclusion. Decrease of SmB and SmD3 mRNA levels does not affect the parasite, indicating that the spliceosome remains functional or that splicing is not essential for parasite viability.
Downloads
References
Ankarklev J, Jerlström-Hultqvist J, Ringqvist E, Troell K, Svärd SG. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol. 2010;8:413-22. http://dx.doi.org/10.1038/nrmicro2317.
Painter JE, Gargano JW, Collier SA, Yoder JS, Centers for Disease Control and Prevention. Giardiasis surveillance – United States , 2011-2012. MMWR. 2015;64(Suppl):15-25.
Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26:58-85. http://dx.doi.org/10.1128/CMR.00067-12.
Lujan HD, Mowatt MR, Byrd LG, Nash TE. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc Natl Acad Sci. 1996;93:7628-33. http://dx.doi.org/10.1073/pnas.93.15.7628.
Sogin M, Gunderson J, Elwood H, Alonso R, Peattie D. Phylogenetic meaning of the kingdom concept: An unusual ribosomal RNA from Giardia lamblia. Science. 1989;243:75-7. http://dx.doi.org/10.1126/science.2911720.
Best AA. Evolution of eukaryotic transcription: Insights from the genome of Giardia lamblia. Genome Res. 2004; 14:1537-47. http://dx.doi.org/10.1101/gr.2256604.
Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921-6. http://dx.doi.org/10.1126/science.1143837.
Nixon JE, Wang A, Morrison HG, McArthur AG, Sogin ML, Loftus BJ, et al. A spliceosomal intron in Giardia lamblia. Proc Natl Acad Sci USA. 2002;99:3701-5. http://dx.doi.org/10.1073/pnas.042700299.
Russell AG, Shutt TE, Watkins RF, Gray MW. An ancient spliceosomal intron in the ribosomal protein L7a gene (Rpl7a) of Giardia lamblia. BMC Evol Biol. 2005;5:45. http://dx.doi.org/10.1186/1471-2148-5-45.
Roy SW, Hudson AJ, Joseph J, Yee J, Russell AG. Numerous fragmented spliceosomal introns, AT-AC splicing, and an unusual dynein gene expression pathway in Giardia lamblia. Mol Biol Evol. 2012;29:43-9. http://dx.doi.org/10.1093/molbev/msr063.
Franzén O, Jerlström-Hultqvist J, Einarsson E, Ankarklev J, Ferella M, Andersson B, et al. Transcriptome profiling of Giardia intestinalis using strand-specific RNA-Seq. PLoS Comput Biol. 2013;9:e1003000. http://dx.doi.org/10.1371/journal.pcbi.1003000.
Kamikawa R, Inagaki Y, Hashimoto T. Secondary loss of a cis-spliced intron during the divergence of Giardia intestinalis assemblages. BMC Res Notes. 2014;7:413. http://dx.doi.org/10.1186/1756-0500-7-413.
Kamikawa R, Inagaki Y, Tokoro M, Roger AJ, Hashimoto T. Split introns in the genome of Giardia intestinalis are excised by spliceosome-mediated trans-splicing. Curr Biol. 2011;21:311-5. http://dx.doi.org/10.1016/j.cub.2011.01.025.
Nageshan RK, Roy N, Hehl AB, Tatu U. Post-transcriptional repair of a split heat shock protein 90 gene by mRNA trans-splicing. J Biol Chem. 2011;286:7116-22. http://dx.doi.org/10.1074/jbc.C110.208389.
Kambach C, Walke S, Young R, Avis JM, de la Fortelle E, Raker VA, et al. Crystal structures of two SM protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell. 1999;96:375-87. http://dx.doi.org/10.1016/S0092-8674(00)80550-4.
Raker VA, Plessel G, Lührmann R. The snRNP core assembly pathway: Identification of stable core protein heteromeric complexes and an snRNP subcore particle in vitro. EMBO J. 1996;15:2256-69.
Collins L, Penny D. Complex spliceosomal organization ancestral to extant eukaryotes. Mol Biol Evol. 2005;22: 1053-66. http://dx.doi.org/10.1093/molbev/msi091.
Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487-8. http://dx.doi.org/10.1016/0035-9203(83)90120-7.
Kane AV, Ward HD, Keusch GT, Pereira ME. In vitro encystation of Giardia lamblia: Large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J Parasitol. 1991;77:974. http://dx.doi.org/10.2307/3282752.
Niño CA, Prucca CG, Chaparro J, Luján HD, Wasserman M. The ubiquitin-activating enzyme (E1) of the early-branching eukaryote Giardia intestinalis shows unusual proteolytic modifications and play important roles during encystation. Acta Trop. 2012;123:39-46. http://dx.doi.org/10.1016/j.actatropica.2012.03.012.
Teodorovic S, Walls CD, Elmendorf HG. Bidirectional transcription is an inherent feature of Giardia lamblia promoters and contributes to an abundance of sterile antisense transcripts throughout the genome. Nucleic Acids Res. 2007;35:2544-53. http://dx.doi.org/10.1093/nar/gkm105.
Prucca CG, Slavin I, Quiroga R, Elías E V, Rivero FD, Saura A, et al. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature. 2008;456:750-4. http://dx.doi.org/10.1038/nature07585.
Alvarado ME, Wasserman M. Calmodulin expression during Giardia intestinalis differentiation and identification of calmodulin-binding proteins during the trophozoite stage. Parasitol Res. 2012;110:1371-80. http://dx.doi.org/10.1007/s00436-011-2637-4.
Elmendorf HG. The abundance of sterile transcripts in Giardia lamblia. Nucleic Acids Res. 2001;29:4674-83. http://dx.doi.org/10.1093/nar/29.22.4674.
Wan L, Battle DJ, Yong J, Gubitz AK, Kolb SJ, Wang J, et al. The survival of motor neurons protein determines the capacity for snRNP assembly: Biochemical deficiency in spinal muscular atrophy. Mol Cell Biol. 2005;25:5543-51. http://dx.doi.org/10.1128/MCB.25.13.5543-5551.2005.
Some similar items:
- Carlos Alberto Niño, Moisés Wasserman, Synthesis of pyruvate: ferredoxin oxidoreductase and alcohol dehydrogenase E enzymes during Giardia intestinalis excystation , Biomedica: Vol. 30 No. 1 (2010)
- Zaava Ravid, Sofía Duque, Adriana Arévalo, Rubén Santiago Nicholls, Moisés Wasserman, Genetic diversity of Giardia intestinalis populations in Colombia , Biomedica: Vol. 27 No. 1 (2007)
- Paula C. Hernández, María Leonor Caldas, Moisés Wasserman, In vitro encystation of Giardia lamblia: analysis with two-dimensional electrophoresis of differentially expressed proteins. , Biomedica: Vol. 22 No. 3 (2002)
- Hector Hernán Henao, Yaneth Osorio, Nancy Gore Saravia, Arlen Gómez, Bruno Travi, Efficacy and toxicity of pentavalent antimonials (Glucantime and Pentostam) in an American cutaneous leishmaniasis animal model: luminometry application. , Biomedica: Vol. 24 No. 4 (2004)
- Yenny Alviarez, María Lares, Mercedes Viettri, Cruz M. Aguilar, Leidi Herrera, Elizabeth Ferrer, Standardization of a direct agglutination test for the immunodiagnosis of Chagas disease , Biomedica: Vol. 34 No. 2 (2014)
- Giovanni Andrés Polo, Carmenza Janneth Benavides, Juan Manuel Astaiza, Dario Antonio Vallejo, Patricia Betancourt, Enteroparasite determination in Lactuca sativa from farms dedicated to its production in Pasto, Colombia , Biomedica: Vol. 36 No. 4 (2016)
- Julián A. Fernández-Niño, Claudia I. Astudillo-García, Laura María Segura, Natalia Gómez, Ángela Skantria Salazar, Juan Hember Tabares, Cristian Andrés Restrepo, Miguel Ángel Ruiz, Myriam Consuelo López, Patricia Reyes, Profiles of intestinal polyparasitism in a community of the Colombian Amazon region , Biomedica: Vol. 37 No. 3 (2017)
- Álvaro Francisco Dulce-Villarreal, Angélica María Rojas-Bárcenas, José Danilo Jojoa-Ríos, José Fernando Gómez-Urrego, Human intestinal myiasis by Eristalis tenax in a child from the urban area of the municipality of Policarpa, Nariño, Colombia , Biomedica: Vol. 40 No. 4 (2020)
- Jorge Iván Zapata-Valencia, Sebastián Ortega-Valencia , Yisther Katherine Silva-Cuero, Lina Sofía Castillo-Castillo , Laura Sofía Ortega-Ruíz , Adriana Cardona-Ortiz , Juliana Peña-Stadlin , Frequency of enteroparasites in Cebidae and Callitrichidae primates at the Zoológico de Cali, Colombia: zoonotic implications , Biomedica: Vol. 41 No. Supl. 1 (2021): Mayo, Parasitología médica
- Alicia Ortega-Narváez, Luis Reinel Vásquez-Arteaga, Olga Cujar-Otero, Jehyson Madroñero Daza, Ginna Cabra-Bautista, Tungiasis in the urban area of Popayán, Colombia: A case report , Biomedica: Vol. 41 No. Supl. 1 (2021): Mayo, Parasitología médica
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























