First evidence of lymphocytic choriomeningitis virus (Arenavirus) infection in Mus musculus rodents captured in the urban area of the municipality of Sincelejo, Sucre, Colombia
Abstract
Introduction: The lymphocytic choriomeningitis virus is an Old World arenavirus that infects Mus musculus, and can cause congenital hydrocephalus, chorioretinitis and multisystemic failure in transplant human recipients. Although the disease has not been clinically diagnosed in Colombia yet, there have been reports of infection with the Pichindé virus in rodents from Cauca and Valle del Cauca departments, and with the Guanarito virus in rodents from Córdoba department.
Objective: To identify the lymphocytic choriomeningitis virus from Mus musculus captured in the municipality of Sincelejo.
Materials and methods: We evaluated 80 samples of plasma by ELISA using antigen from lymphocytic choriomeningitis virus. Additionally, a nested RT-PCR was performed to seropositive and seronegative samples for the S-segment.
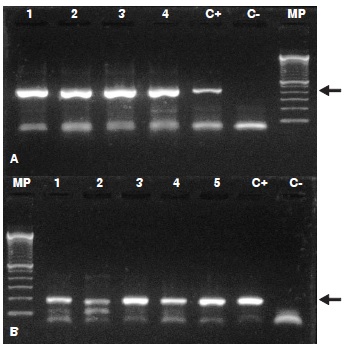
Results: We found a 10% seroprevalence (8/80) and the viral genome was detected in 16 brain samples; the alignment (BLAST) and the phylogenetic analysis (MrBayes, version 3.2.2) confirmed the presence of the lymphocytic choriomeningitis virus.
Conclusion: The results indicated that human infection with the lymphocytic choriomeningitis virus in humans could occur in the urban area of Sincelejo, although no cases have been reported so far.
Downloads
References
York J, Berry JD, Stroher U, Li Q, Feldmann H, Lu M, et al. An antibody directed against the fusion peptide of Junin virus envelope glycoprotein GPC inhibits pH-induced membrane fusion. J Virol. 2010;84:6119-29. http://dx.doi.org/10.1128/JVI.02700-09
Machado AM, Figueiredo GG, Campos GM, Lozano ME, Machado AR, Figueiredo LT. Standardization of an ELISA test using a recombinant nucleoprotein from the Junin virus as the antigen and serological screening for arenavirus among the population of Nova Xavantina, State of Mato Grosso. Rev Soc Bras Med Trop. 2010;43:229-33. http://dx.doi.org/10.1590/S0037-86822010000300003
Charrel RN, de Lamballerie X, Emonet S. Phylogeny of the genus Arenavirus. Curr Opin Microbiol. 2008;11:362-8. http://dx.doi.org/10.1016/j.mib.2008.06.001
Charrel RN, de Lamballerie X. Zoonotic aspects of arenavirus infections. Vet Microbiol. 2010;140:213-20. http://dx.doi.org/10.1016/j.vetmic.2009.08.027
Zapata JC, Salvato SM. Arenavirus variations due to hostspecific adaptation. Viruses. 2013;5:241-78. http://dx.doi.org/10.3390/v5010241
Childs JE, Kaufmann AF, Peters CJ, Ehrenberg RL. Hantavirus infection--southwestern United States: Interim recommendations for risk reduction. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1993;42:1-13.
Trapido H, Sanmartín C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg. 1971;20:631-41.
Mattar S, Guzmán C, Arrázola J, Soto E, Barrios J, Pini N, et al. Antibody to arenaviruses in rodents, Caribbean Colombia. Emerg Infect Dis. 2011;17:1315-7. http://dx.doi.org/10.3201/eid1707.101961
Arrázola-D J, Londoño A, Arroyabe E, Rodas J, Salazar-Bravo J, Mattar-VS. Vigilancia del virus de la coriomeningitis linfocítica (LCMV) en roedores reservorios de Córdoba (Colombia). Rev Méd Evidencias. 2014;3:5-14.
Mills J, Childs J, Ksiazek T, Peters CJ, Velleca WM. Methods for trapping and sampling small mammals for virologic testing. Atlanta, GA: CDC; 1998.
Nowak R. Walker`s Mammals of the World. Sixth edition. Baltimore: The Johns Hopkins University Press; 1999. p.1-2015.
Ledesma J, Fedele CG, Carro F, Lledó L, Sánchez-Seco MP, Tenorio A, et al. Independent lineage of lymphocytic choriomeningitis virus in wood mice (Apodemus sylvaticus), Spain. Emerg Infect Dis. 2009;15:1677-80. http://dx.doi.org/10.3201/eid1510.090563
Smith LM, Sanders JZ, Kaiser RJ, Hughes P, Dodd C, Connell CR, et al. Fluorescence detection in automated DNA sequence analysis. Nature.1986;321:674-9. http://dx.doi.org/10.1038/321674a0
Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Biochemistry. 1977;74:5463-7.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-80. http://dx.doi.org/10.1093/nar/22.22.4673
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731-9. http://dx.doi.org/10.1093/molbev/msr121.
Macneil A, Stroher U, Farnon E, Campbell S, Cannon D, Paddock CD, et al. Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg Infect Dis. 2012;18:1256-62. http://dx.doi.org/10.3201/eid1808.120212
Kallio-Kokko H, Laakkonen J, Rizzoli A, Tagliapietra V, Cattadori I, Perkins SE, et al. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol Infect. 2006;134:830-6. http://dx.doi.org/10.1017/S0950268805005431
Riera L, Castillo E, Del Carmen-Saavedra M, Priotto J, Sottosanti J, Polop J, et al. Serological study of the lymphochoriomeningitis virus (LCMV) in an inner city of Argentina. J Med Virol. 2005;76:285-9. http://dx.doi.org/10.1002/jmv.20357
Glass GE, Shields T, Cai B, Yates TL, Parmenter R. Persistently highest risk areas for hantavirus pulmonary syndrome: Potential sites for refugia. Ecol Appl. 2007;17:129-39.
Madhav NK, Wagoner KD, Douglass RJ, Mills JN. Delayed density-dependent prevalence of Sin Nombre virus antibody in Montana deer mice (Peromyscus maniculatus) and implications for human disease risk. Vector Borne Zoonotic Dis. 2007;7:353-64. http://dx.doi.org/10.1089/vbz.2006.0605
Lehmer E, Clay C, Pearce-Duvet J, St Jeor S, Dearing M. Differential regulation of pathogens: The role of habitat disturbance in predicting prevalence of Sin Nombre virus. Oecologia. 2008;155:429-39. http://dx.doi.org/10.1007/s00442-007-0922-9
Tersago K, Schreurs A, Linard C, Verhagen R, van Dongen S, Leirs H. Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector Borne Zoonotic Dis. 2008;8:235-44. http://dx.doi.org/10.1089/vbz.2007.0160
Suzan G, Marce E, Giermakowski JT, Armien B, Pascale J, Mills J, et al. The effect of habitat fragmentation and species diversity loss on hantavirus prevalence in Panamá. Ann NY Acad Sci. 2008;1149:80-3. http://dx.doi.org/10.1196/annals.1428.063
Kuenzi AJ, Douglass RJ, Bond CW, Calisher CH, Mills JN. Long-term dynamics of Sin Nombre viral RNA and antibody in deer mice in Montana. J Wildl Dis. 2005;41:473-81. http://dx.doi.org/10.7589/0090-3558-41.3.473
Hjelle B, Glass GE. Outbreak of hantavirus infection in the Four Corners region of the United States in the wake of the 1997-1998 El Niño-southern oscillation. J Infect Dis. 2000;181:1569-73. http://dx.doi.org/1010.1086/315467
Fischer SA, Graham MB, Kuehnert MJ, Kotton CN, Srinivasan A, Marty FM, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354:2235-49. http://dx.doi.org/10.1056/NEJMoa053240
Buchmeier MJ, Peters CJ. Arenaviridae: The viruses and their replication. 5th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. p. 1791-828.
Jamieson D, Bell M, Rasmussen S, Kourtis A. Lymphocytic choriomeningitis virus: An emerging obstetric pathogen? Am J Obstet Gynecol 2006;194:1532-6. http://dx.doi.org/10.1016/j.ajog.2005.11.040
Takagi T, Ohsawa M, Morita C, Sato, H, Ohsawa K. Genomic analysis and pathogenic characteristics of lymphocytic choriomeningitis virus strains isolated in Japan. Comp Med. 2012;62:185-92.
Musser G, Carleton M. Mammal species of the world: A taxonomic and geographic reference: Superfamily Muridae. 3rd edition. Washington, DC: Smithsonian Institution; 2005. p. 894-1531.
Tagliapietra V, Rosa R, Hauffe HC, Laakkonen J, Voutilainen L, Vapalahti O, et al. Spatial and temporal dynamics of lymphocytic choriomeningitis virus in wild rodents, northern Italy. Emerg Infect Dis. 2009;15:1019-25. http://dx.doi.org/10.3201/eid1507.081524
Zinkernagel RM. Immunity, immunopathology and vaccines against HIV? Vaccine. 2002;20:1913-7. http://dx.doi.org/10.1016/S0264-410X(02)00066-X
Peters CJ, Wilson MR. Diseases of the central nervous system caused by lymphocytic choriomeningitis virus and other arenaviruses. Handb Clin Neurol. 2014;123:671-81. http://dx.doi.org/10.1016/B978-0-444-53488-0.00033-X
King CC, Jamieson BD, Reddy K, Bali N, Concepcion RJ, Ahmed R. Viral infection of the thymus. J Virol. 1992;66:3155-60.
Oldstone MB, Frank FJ. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969;129:483-505.
Rodríguez M, Buchmeier MJ, Oldstone MB, Lampert PW. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Am J Pathol. 1983;110:95-100.
Nathanson N, Ahmed R, Biron C, Briton M, González-Scarano F, Griffin D, et al. Viral pathogenesis and immunity. Second edition. California, USA: Academic Press; p. 2007. p . 265.
Heeney JL. Zoonotic viral diseases and the frontier of early diagnosis, control and prevention. J Intern Med. 2006;260:399-408. http://dx.doi.org/10.1111/j.1365-2796. 2006.01711.x
Mets MB, Barton LL.Congenital lymphocytic choriomeningitis virus infection: Decade of rediscovery. Clin Infect Dis. 2006;33:70-4. http://dx.doi.org/10.1086/321897
Some similar items:
- Ana María Perilla, Camilo González, Sandra Liliana Valderrama, Natasha Vanegas, Bibiana Chavarro, Luis Carlos Triana, José Roberto Támara, Carlos Arturo Álvarez, Necrotizing pneumonia by community-acquired, methicillin-resistant Staphylococcus aureus in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Yolanda Lucía López, Claudia González, Berta Natalia Gallego, Ana Lida Moreno, Stewardship of public health surveillance in the health system in Colombia: a cases study , Biomedica: Vol. 29 No. 4 (2009)
- Oscar Fernando Herrán, María F. Ardila, Categories of alcohol consumers and the criteria for classification , Biomedica: Vol. 29 No. 4 (2009)
- Ingrid Yamile Pulido, José Ramón Mantilla, Emilia María Valenzuela, María Teresa Reguero, Elsa Beatriz González, Distribution of extended spectrum β-lactamases-codifying genes in Klebsiella pneumoniae isolates from hospitals of Bogota, D.C., Colombia , Biomedica: Vol. 31 No. 1 (2011)
- Greizy López, Nancy Yaneth Gelvez, Martalucía Tamayo, Mutational frequencies in usherin (USH2A gene) in 26 Colombian individuals with Usher syndrome type II , Biomedica: Vol. 31 No. 1 (2011)
- Jhon Carlos Castaño, Fidel Ángel Núñez, María Mercedes González, Germán Téllez, María Isabel Giraldo, First case report of Mammomonogamus (Syngamus) laryngeus human infection in Colombia , Biomedica: Vol. 26 No. 3 (2006)
- Andrés F. Londoño, Silvana Levis, Juan D. Rodas, Hantavirus as important emerging agents in South America , Biomedica: Vol. 31 No. 3 (2011)
- Jefferson Antonio Buendía, Attitudes, knowledge and beliefs of patient about anti-hypertensive drugs , Biomedica: Vol. 32 No. 4 (2012)
- Mauricio Beltrán, Maritza Berrío-Pérez, María Isabel Bermúdez, Gloria Rey-Benito, Bernardo Camacho, Patricia Forero, Gloria Cristina Molina, Orlando Fals, Isabel Pisciotti, Yulieth Oliveros, Armando Cortés, Fernando De La Hoz, Absence of occult hepatitis B in Colombian blood donors , Biomedica: Vol. 31 No. 4 (2011)
- Pablo Chaparro, Edison Soto, Julio Padilla, Daniel Vargas, Estimation of the underreporting of malaria measurement in ten municipalities of the Pacific coast of Nariño during 2009 , Biomedica: Vol. 32 (2012): Suplemento 1, Malaria
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























