Mutagenicity, genotoxicity and gene expression of Rad51C, Xiap, P53 and Nrf2 induced by antimalarial extracts of plants collected from the middle Vaupés region, Colombia
Abstract
Introduction: Due to Plasmodium resistance to antimalarial drugs, it is important to find new therapeutic alternatives for malaria treatment and control. Based on the knowledge of Colombian indigenous communities, we collected extracts of plants with potential antimalarial effects from the middle Vaupés region.
Objective: To evaluate the mutagenic and genotoxic effects, as well as the gene expression of Rad51C, Xiap, P53 and Nrf2 induced by four ethanolic extracts with antimalarial activity (R001, T002, T015 and T028).
Materials and methods: We evaluated four ethanolic extracts with antimalarial activity using the Ames test to assess mutagenicity, and the comet assay on HepG2 cells to determine the genotoxicicity. We also evaluated the expression of Rad51C, Xiap, P53 and Nrf2 from HepG2 cells stimulated with the four extracts.
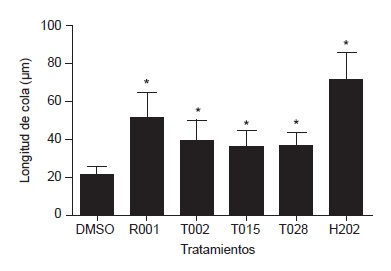
Results: None of the four extracts was mutagenic in Salmonella typhimurium TA98 strain in the presence and absence of S9 metabolic activity. Extracts R001, T015 and T028 were weakly mutagenic on the TA100 strain in the presence of S9, with mutagenic indexes (MI) of 1.58, 1.53 and 1.61, respectively. The T015 strain showed the same behavior without S9 with an MI of 1.36. The results of the comet assay showed that the four extracts produced category 1 or 2 damage, with comets between 36.7 and 51.48 μm in length. However, the genetic damage index suggested that most of the cells were affected by the treatments. Regarding gene expression, extracts R001 and T028 induced an overexpression of genes Xiap and P53 with an 1.84 to 3.99 fold-change compared with untreated cells.
Conclusions: These results revealed that the T002 extract was the safest as it had antimalarial activity and was not cytotoxic on HepG2 cells. Moreover, it was not mutagenic and it only produced category 1 damage on the DNA. Also, the extract did not induce a change in the expression of the tested genes.
Downloads
References
World Health Organisation. World malaria report, 2014. Fecha de consulta: 14 de junio de 2016. Disponible en:
http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/
Gligorijevic B, Purdy K, Elliott DA, Cooper RA, Roepe PD. Stage independent chloroquine resistance and chloroquine toxicity revealed via spinning disk confocal microscopy. Mol Biochem Parasitol. 2008;159:7-23. https://doi.org/10.1016/j.molbiopara.2007.12.014
Ramírez MO, Cardona NF, Pabón VA, Blair TS. Etnobotánica de las plantas antimaláricas del Vaupés Medio: recuperación del saber médico tradicional. Actu Biol. 2012;34:154.
Dike IP, Obembe OO, Adebiyi FE. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J Ethnopharmacol. 2012;144:618-26. https://doi.org/10.1016/j.jep.2012.10.002
Murambiwa P, Masola B, Govender T, Mukaratirwa S, Musabayane CT. Anti-malarial drug formulations and novel delivery systems: A review. Acta Trop. 2011;118:71-9. https://doi.org/10.1016/j.actatropica.2011.03.005
Oksman-Caldentey K-M, Inzé D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004;9:433-40. https://doi.org/10.1016/j.tplants.2004.07.006
Pabón A, Ramírez O, Ríos A, López E, de Las Salas B, Cardona F, et al. Antiplasmodial and cytotoxic activity of raw plant extracts as reported by knowledgeable indigenous people of the Amazon region (Vaupés Medio in Colombia). Planta Med. 2016;82:717-22. https://doi.org/10.1055/s-0042-104283
S. Flückiger-Isler MK. The Ames MPFTM 98/100 assay: Novel mutagenicity testing in liquid microplate format using S. typhimurium TA98 and TA100. Gewerbestrasse: Xenometrix; 2006.
Westerink WM, Stevenson JC, Horbach GJ, Schoonen WG. The development of RAD51C, Cystatin A, p53 and Nrf2 luciferase-reporter assays in metabolically competent HepG2 cells for the assessment of mechanism-based genotoxicity and of oxidative stress in the early research phase of drug development. Mutat Res. 2010;696:21-40. https://doi.org/10.1016/j.mrgentox.2009.12.007
García-Huertas P, Pabón A, Arias C, Blair S. Evaluación del efecto citotóxico y del daño genético de extractos estandarizados de Solanum nudum con actividad antiplasmodial. Biomédica. 2013;33:78-87. https://doi.org/10.7705/biomedica.v33i1.838
Mayence A, Vanden-Eynde JJ, Kaiser M, Brun R, Yarlett N, Huang TL. Bis(oxyphenylene)benzimidazoles: A novel class of anti-Plasmodium falciparum agents. Bioorg Med Chem. 2011;19:7493-500. https://doi.org/10.1016/j.bmc.2011.10.039
Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173-215. https://doi.org/10.1016/0165-1161(83)90010-9
Flückiger-Isler S, Kamber M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat Res. 2012;747:36-45. https://doi.org/10.1016/j.mrgentox.2012.03.014
Platel A, Gervais V, Sajot N, Nesslany F, Marzin D, Claude N. Study of gene expression profiles in TK6 human cells exposed to DNA-oxidizing agents. Mutat Res. 2010;689:21-49. https://doi.org/10.1016/j.mrfmmm.2010.04.004
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184-91. https://doi.org/10.1016/0014-4827(88)90265-0
Zúñiga-Venegas LA, Creus-Capdevila A, Marcos-Dauder R. Optimizaciones metodológicas del ensayo del cometa y su aplicación en biomonitorización humana. Barccelona: Universitat Autònoma de Barcelona; 2009.
Guilherme S, Gaivão I, Santos MA, Pacheco M. European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup--a glyphosatebased herbicide. Mutagenesis. 2010;25:523-30. https://doi.org/10.1093/mutage/geq038
Rodrigues CD, Hannus M, Prudêncio M, Martin C, Gonçalves LA, Portugal S, et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe. 2008;4:271-82. https://doi.org/10.1016/j.chom.2008.07.012
Verma N, Pink M, Rettenmeier AW, Schmitz-Spanke S. Benzo[a]pyrene-mediated toxicity in primary pig bladder epithelial cells: A proteomic approach. J Proteomics. 2013;85:53-64. https://doi.org/10.1016/j.jprot.2013.04.016
Chen M, Gu H, Ye Y, Lin B, Sun L, Deng W, et al. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem Toxicol. 2010;48:2980-7. https://doi.org/10.1016/j.fct.2010.07.037
Minicucci EM, Ribeiro DA, da Silva GN, Pardini MI, Montovani JC, Salvadori DM.The role of the TP53 gene during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. Exp Toxicol Pathol. 2011;63:483-9. https://doi.org/10.1016/j.etp.2010.03.009
Amiri F, Zarnani A-H, Zand H, Koohdani F, Jeddi-Tehrani M, Vafa M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur J Pharmacol. 2013;718:34-40. https://doi.org/10.1016/j.ejphar.2013.09.020
Mukherjee A, Misra S, Howlett NG, Karmakar P. Multinucleation regulated by the Akt/PTEN signaling pathway is a survival strategy for HepG2 cells. Mutat Res. 2013;755:135-40. https://doi.org/10.1016/j.mrgentox.2013.06.009
Murugaiyan J, Rockstroh M, Wagner J, Baumann S, Schorsch K, Trump S, et al. Benzo[a]pyrene affects Jurkat T cells in the activated state via the antioxidant response element dependent Nrf2 pathway leading to decreased IL-2 secretion and redirecting glutamine metabolism. Toxicol Appl Pharmacol. 2013;269:307-16. https://doi.org/10.1016/j.taap.2013.03.032
Das PJ, Paria N, Gustafson-Seabury A, Vishnoi M, Chaki SP, Love CC, et al. Total RNA isolation from stallion sperm and testis biopsies. Theriogenology. 2010;74:1099-106. https://doi.org/10.1016/j.theriogenology.2010.04.023
Viegas O, Žegura B, Pezdric M, Novak M, Ferreira IM, Pinho O, et al. Protective effects of xanthohumol against the genotoxicity of heterocyclic aromatic amines MeIQx and PhIP in bacteria and in human hepatoma (HepG2) cells. Food Chem Toxicol. 2012;50:949-55. https://doi.org/10.1016/j.fct.2011.11.031
Stephenson FH. Calculations for molecular biology and biotechnology. A guide to mathematics in the laboratory.
Second edition. London: Academic Press; 2010. p. 458.
Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101:95-9. https://doi.org/10.1016/j.jep.2005.03.011
Ahir BK, Pratten MK. Developmental cardiotoxicity effects of four commonly used antiepileptic drugs in embryonic chick heart micromass culture and embryonic stem cell culture systems. Toxicol In Vitro. 2014;28:948-60. https://doi.org/10.1016/j.tiv.2014.04.001
Darwish W, Ikenaka Y, Eldaly E, Ishizuka M. Mutagenic activation and detoxification of benzo[a]pyrene in vitro by hepatic cytochrome P450 1A1 and phase II enzymes in three meat-producing animals. Food Chem Toxicol. 2010;48:2526-31. https://doi.org/10.1016/j.fct.2010.06.026
Zeiger E. Historical perspective on the development of the genetic toxicity test battery in the United States. Environ Mol Mutagen. 2010;51:781-91. https://doi.org/10.1002/em.20602
Rodrigues S, Antunes SC, Correia AT, Nunes B. Acute and chronic effects of erythromycin exposure on oxidative stress and genotoxicity parameters of Oncorhynchus mykiss. Sci Total Environ. 2016;545-546:591-600. https://doi.org/10.1016/j.scitotenv.2015.10.138
Madikizela B, Ndhlala AR, Finnie JF, van Staden J. Antimycobacterial, anti-inflammatory and genotoxicity evaluation of plants used for the treatment of tuberculosis and related symptoms in South Africa. J Ethnopharmacol. 2014;153:386-91. https://doi.org/10.1016/j.jep.2014.02.034
Renglin-Lindh A, Schultz N, Saleh-Gohari N, Helleday T. RAD51C (RAD51L2) is involved in maintaining centrosome number in mitosis. Cytogenet Genome Res. 2007;116:38-45. https://doi.org/10.1159/000097416
de Almagro MC, Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol. 2012;34:200-11.
Sun M, Meares G, Song L, Jope RS. XIAP associates with GSK3 and inhibits the promotion of intrinsic apoptotic signaling by GSK3. Cell Signal. 2009;21:1857-65. https://doi.org/10.1016/j.cellsig.2009.08.002
Boehme K, Dietz Y, Hewitt P, Mueller SO. Activation of P53 in HepG2 cells as surrogate to detect mutagens and promutagens in vitro. Toxicol Lett. 2010;198:272-81. https://doi.org/10.1016/j.toxlet.2010.07.007
Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705-17. https://doi.org/10.1016/j.bcp.2012.11.016
Klaassen CD, Reisman SA. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57-65. https://doi.org/10.1016/j.taap.2010.01.013
Stępkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med. 2011;50:1186-95. https://doi.org/10.1016/j.freeradbiomed.2011.01.033
Roy A, Sil PC. Taurine protects murine hepatocytes against oxidative stress-induced apoptosis by tert-butyl hydroperoxide via PI3K/Akt and mitochondrial-dependent pathways. Food Chem. 2012;131:1086-96. https://doi.org/10.1016/j.foodchem.2011.09.057
Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res. 2015;779:33-45. https://doi.org/10.1016/j.mrfmmm.2015.06.007
Slamenova D, Kozics K, Hunakova L, Melusova M, Navarova J, Horvathova E. Comparison of biological processes induced in HepG2 cells by tert-butyl hydroperoxide (t-BHP) and hydroperoxide (H2O2): The influence of carvacrol. Mutat Res. 2013;757:15-22. https://doi.org/10.1016/j.mrgentox.2013.03.014
International Agency for Research on Cancer -IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. 2010;92:35-773.
Cheng T, Scadden DT. Cell cycle regulators in stem cells. In: Lanza R, Atala A, editors. Essentials of Stem Cell Biology. Third edition. Boston: Academic Press; 2014. p. 95-106. https://doi.org/10.1016/B978-0-12-409503-8.00008-1
Tyson JJ, Novák B. Irreversible transitions, bistability and checkpoint controls in the eukaryotic cell cycle: A systemslevel understanding. In: Walhout AJM, Vidal M, Dekker J, editors. Handbook of Systems Biology. San Diego: Elsevier; 2013. p. 265-85. https://doi.org/10.1016/B978-0-12-385944-0.00014-9
Some similar items:
- Iveth J. González, Metacaspases and their role in the life cycle of human protozoan parasites , Biomedica: Vol. 29 No. 3 (2009)
- Rosa Magdalena Uscátegui, Adriana M. Correa, Jaime Carmona-Fonseca, Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria , Biomedica: Vol. 29 No. 2 (2009)
- Ana María Vásquez, Felipe Sanín, Luis Gonzalo Álvarez, Alberto Tobón, Alexandra Ríos, Silvia Blair, Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development , Biomedica: Vol. 29 No. 2 (2009)
- Alberto Tobón, Danger signs in the malaria patient , Biomedica: Vol. 29 No. 2 (2009)
- Juan Gabriel Piñeros, Margarita Arboleda, Juan Camilo Jaramillo, Silvia Blair, Report of five cases of severe neonatal Plasmodium vivax malaria in Urabá, Colombia , Biomedica: Vol. 28 No. 4 (2008)
- Amanda Maestre, Jaime Carmona-Fonseca, Amanda Maestre, Alta frecuencia de mutaciones puntuales en pfcrt de Plasmodium falciparum y emergencia de nuevos haplotipos mutantes en Colombia , Biomedica: Vol. 28 No. 4 (2008)
- Silvia Blair, Ana Mercedes Rada, Carolina Moreno, Successful in vitro culture of Plasmodium falciparum gametocytes , Biomedica: Vol. 28 No. 4 (2008)
- Jaime Carmona-Fonseca, Eliana Arango, Silvia Blair, Gametocytemia in falciparum malaria treated with amodiaquine or artesunate , Biomedica: Vol. 28 No. 2 (2008)
- Angélica Knudson, Rubén Santiago Nicholls, Ángela Patricia Guerra, Ricardo Sánchez, Clinical profiles of patients with uncomplicated Plasmodum falciparum malaria in northwestern Colombia , Biomedica: Vol. 27 No. 4 (2007)
- Paula Montoya, Alberto Tobón, Silvia Blair, Jaime Carmona, Amanda Maestre, Polymorphisms of the pfmdr1 gene in field samples of Plasmodium falciparum and their association with therapeutic response to antimalarial drugs and severe malaria in Colombia , Biomedica: Vol. 27 No. 2 (2007)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























