Standardization of a multiplex real-time PCR test for the identification of Angiostrongylus cantonensis, A. costaricensis and A. vasorum
Abstract
Introduction: Angiostrongyliasis is a disease caused by Angiostrongylus nematodes that is present worldwide. The infections with the highest impact on human and animal health are caused by A. cantonensis, A. costaricensis, and A. vasorum. Clinical forms of the disease in humans are eosinophilic meningitis and abdominal angiostrongyliasis, while the most common effect on dogs are cardiopulmonary damages. It is deemed as an emerging disease as the result of the global dissemination of the African snail Lissachatina fulica, an intermediary host of these parasites. The few diagnostic methods for Angiostrongylus spp. are unspecific, costly, and not very sensitive. It is urgent to develop a sensitive, specific and accessible diagnostic tool for the control of human and animal angiostrongyliasis.
Objective: To develop a qPCR multiple test to identify the three pathogenic species of Angiostrongylus.
Materials and methods: Through a bio-informatic analysis, we selected a sequence of the ITS-2 region of the Angiostrongylus genome to guarantee the specificity of primers and probes. We extracted DNA from adult parasites as positive control, and from larvae using the DNeasy Blood&Tissue® kit. Quantitative PCR reactions were conducted on a Smartcycler Cepheid® thermocycler using a master mix QuantiTect® kit. DNA from human beings, other parasites and the African snail was used as negative control.
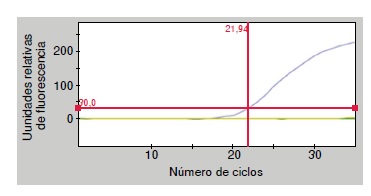
Results: The threshold cycle values for positive DNA controls were: 21 for Angiostrongylus cantonensis, 22 for A. costaricensis, and 31 for A. vasorum. In negative controls, the threshold cycle was zero. qPCR showed an amplification efficiency of 2 (100%).
Conclusions: A multiple qPCR was standardized at the laboratory for three clinically significant species of Angiostrongylus.
Downloads
References
Maldonado Jr A, Simões R, Thiengo S. Angiostrongyliasis in the Americas. Zoonosis. En: Lorenzo-Morales J, editor. InTech; 2012. p. 303-20. https://doi.org/10.5772/38632
Caldeira LR, Carvalho SO, Mendoça LFG C, Graeff-Teixeira C, Silva CF M, Ben R, et al. Molecular differentiation of Angiostrongylus costaricensis, A. cantonensis, and A. vasorum by polymerase chain reaction-restriction fragment length polymorphism. Mem Inst Oswaldo Cruz. 2003;98:1039-43. https://doi.org/10.1590/S0074-02762003000800011
Morera P, Céspedes R. Angiostrongylus costaricensis n. sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in a man in Costa Rica. Rev Biol Trop. 1970;18:173-85.
Incani RN, Caleiras E, Martín M, González C. Human infection by Angiostrongylus costaricensis in Venezuela: First report of a confirmed case. Rev Inst Med Trop Sao Paulo. 2007;9:197-200. https://doi.org/10.1590/S0036-46652007000300012
Pincay T, García L, Narváez E, Decker O, Martini L, Moreira JM. Angiostrongyliasis due to Parastrongylus (Angiostrongylus) cantonensis in Ecuador. First report in South America. Trop Med Int Health. 2009;14(Suppl.2):37.
Thiengo SC, Maldonado A, Mota EM, Torres EJ, Caldeira R, Carvalho OS, et al. The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop. 2010;115:194-9. https://doi.org/10.1016/j.actatropica.2010.01.005
Cowie RH. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health. 2013;72:6-9.
Bourque A, Conboy G, Miller L, Whitney H, Ralhan S. Angiostrongylus vasorum infection in two dogs from Newfoundland. Can Vet J. 2002;43:876-9.
Qvarnstrom Y, Sullivan JJ, Bishop HS, Hollingsworth R, da Silva AJ. PCR-based detection of Angiostrongylus cantonensis in tissue and mucus secretions from molluscan host. Appl Environ Microbiol. 2007;73:1415-9. https://doi.org/10.1128/AEM.01968-06
Moreira VL, Giese EG, Melo FT, Simões RO, Thiengo SC, Maldonado A Jr, et al. Endemic angiostrongyliasis in the Brazilian Amazon: Natural parasitism of Angiostrongylus cantonensis in Rattus rattus and R. Norvegicus, and sympatric giant African land snail, Achatina fulica. Acta Trop. 2012;125:90-7. https://doi.org/10.1016/j.actatropica.2012.10.001
Kim JR, Hayes KA, Yeung NW, Cowie RH. Diverse gastropod host of Angiostrongylus cantonensis, the rat lungworm globally and with a focus on the Hawaiian Islands. PLoS One. 2014;9:e94969. https://doi.org/10.1371/journal.pone.0094969
Qvarnstrom Y, da Silva AC, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, et al. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1 based Taqman assay. Appl Environ Microbiol. 2010;76:5287-9. https://doi.org/10.1128/AEM.00546-10
Abrahams SE. Angiostrongiliasis abdominal: notas sobre el diagnóstico. Rev Biomed. 2007;18:37-45.
Rodríguez G. Hematoquecia letal por angiostrongilosis abdominal. Biomédica. 2000;20:120-30. https://doi.org/10.7705/biomedica.v20i2.1055
Mota EM, Lenzi HL. Angiostrongylus costaricensis life cycle: A new proposal. Mem Inst. Oswaldo Cruz. 1995;90:707-9. https://doi.org/10.1590/S0074-02761995000600010
Morgan ER, Shaw SE, Brennan SF, De Waal TD, Jones BR, Mulcahy G. Angiostrongylus vasorum a real heartbreaker. Trends Parasitol. 2005;21:49-51. https://doi.org/10.1016/j.pt.2004.11.006
Conboy G. Canine angiostrongylosis (French heartworm). In: Bowman DD, editor. Companion and exotic animal parasitology. Ithaca, NY: International Veterinary Information System; 2000. p. 5.
Coaglio Silva LA. Susceptibilidade e comportamento de Achatina fulica infectada com Angiostrongylus vasorum (tesis). Belo Horizonte: Universidade Federal de Minas Gerais; 2013.
Al-Sabi MN, Deplazes P, Webster P, Willesen JL, Davidson RK, Kapel CM. PCR Detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol Res. 2010;107:135-40. https://doi.org/10.1007/s00436-010-1847-5
Bolt G, Monrad J, Koch J, Jensen AL. Canine angiostrongilosis: A review. Vet Rec. 1994;135:447-52.
Anaya JM, Shoenfeld Y, Correa PA, García-Carrasco M, Cervera R. Autoinmunidad y enfermedad autoinmune. Medellín: Corporación para Investigaciones Biológicas; 2005. p. 515-22.
Rodríguez IP, Barrera HA. La reacción en cadena de la polimerasa a dos décadas de su invención. Ciencia UANL. 2004;7:323-35
Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000;13:559-70. https://doi.org/10.1128/CMR.13.4.559-570.2000
Chang JH, Yen CM, Chen ER, Chung LY, Wang JJ, Chye SM, et al. Detection of antibodies to surface antigens of Angiostrongylus cantonensis by ELISA. Ann Trop Med Parasitol. 1995;89:569-72.
Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Resolución Número 654 del 7 de abril de 2011. “Por la cual se corrige la Resolución No. 0848 del 23 de mayo de 2008
y se adoptan las medidas que deben seguir las autoridades ambientales, para la prevención, control y manejo de la especie caracol gigante africano (Achatina fulica). Bogotá:
Minambiente; 2011.
Qvarnstrom Y, Xayavong M, da Silva AC, Park SY, Whelen AC, Calimlim PS, et al. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am J Trop Med Hyg. 2016;94:176-81. https://doi.org/10.4269/ajtmh.15-0146
Pinilla G, Cubillos K, Rodríguez M. Bodas de plata de la reacción en cadena de la polimerasa (PCR). NOVA. 2008;6:65-75.
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods. 2001;25:386-401. https://doi.org/10.1006/meth.2001.1261
Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH. Multiplex PCR: Critical parameters and step-by-step protocol. Biotechniques. 1997;23:504-11.
Markoulatos P, Siafakas N, Moncany M. Multiplex polymerase chain reaction: A practical approach. J Clin Lab Anal. 2002;16:47-51.
Caldillá JS, Paz AA, Fernández-de Mera JJ, Galarraga-Inza J, Cruz-Guerrero G. Meningoencefalomielitis por Angiostrongylus cantonensis con afectación pulmonar. An Esp Pediatr. 1998;49:308-10.
Sauerbrey M. A precipitin test for the diagnosis of human abdominal angiostrongyliasis. Am J Trop Med Hyg. 1977;26:1156-8.
Wei FR, Liu HX, Lv S, Hu L, Zhang Y. Multiplex PCR assay for the detection of Angiostrongylus cantonensis larvae in Pomacea canaliculata. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2010;28:355-8.
Jarvi SI, Farías ME, Howe K, Jacquier S, Hollingsworth R, Pitt W. Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi). Mol Biochem Parasitol. 2012;185:174-6. https://doi.org/10.1016/j.molbiopara.2012.08.002
Eamsobhana P, Wanachiwanawin D, Dechkum N, Parsartvit A, Yong HS. Molecular diagnosis of eosinophilic meningitis due to Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) by polymerase chain reaction-DNA sequencing of cerebrospinal fluids of patients. Mem Inst Oswaldo Cruz. 2013;108:116-8. https://doi.org/10.1590/S0074-02762013000100020
Graeff-Teixeira C, Agostini AA, Camillo-Coura L, Ferreira-da Cruz MF. Seroepidemiology of abdominal angiostrongyliasis: The standardization of an immunoenzymatic assay and prevalence of antibodies in two localities in Southern Brazil. Trop Med Int Health. 1997;2:254-60. https://doi.org/10.1046/j.1365-3156.1997.d01-266.x
Geiger SM, Laitano AC, Sievers-Tostes C, Agostini AA, Schulz-Key H, Graeff-Teixeira C. Detection of the acute phase of abdominal angiostrongyliasis with a parasite specific IgG enzyme linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2001;96:515-8. https://doi.org/10.1590/S0074-02762001000400012
Abraham SE, Schulz-Key H, Geiger S. Caracterización de antígenos de bajo peso molecular de Angiostrongylus costaricensis reconocidos durante una infección experimental en roedores. Parasitol Latinoam. 2004;59:8-13. https://doi.org/10.4067/S0717-77122004000100002
Abrahams SE, Hoffmann WH, Graeff-Teixeira C, Schulz-Key H, Geiger SM. Long-term observations on mouse strains experimentally infected with Angiostrongylus costaricensis. Parasitol Res. 2004;93:230-4. https://doi.org/10.1007/s00436-004-1108-6
Silva AC, Graeff-Teixeira C, Zaha A. Diagnosis of abdominal angiostrongiliasis by PCR from sera of patients. Rev Inst Med Trop Sao Paulo. 2003;45:295-7. https://doi.org/10.1590/S0036-46652003000500011
Di Cesare A, Traversa D. Canine angiostrongylosis: Recent advances in diagnosis, prevention, and treatment. Vet Res. 2014;5:181-92. https://doi.org/10.2147/VMRR.S53641
Miglio A, Antognoni MT, Mangili V. What is your diagnosis? Bronchoalveolar lavage and cerebrospinal fluid from a dog in Italy. Vet Clin Pathol. 2013;42:109-10. https://doi.org/10.1111/vcp.12000
Helm J, Gilleard JS, Jackson M, Redman E, Bell R. A case of canine Angiostrongylus vasorum in Scotland confirmed by PCR and sequence analysis. J Small Anim Pract. 2009;50:255-9. https://doi.org/10.1111/j.1748-5827.2009.00741.x
Jefferies R, Morgan ER, Shaw SE. A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet Parasitol. 2009;166:112-8. https://doi.org/.1016/j.vetpar.2009.07.042
Jefferies R, Morgan ER, Helm J, Robinson M, Shaw SE. Improved detection of canine Angiostrongylus vasorum infection using real-time PCR and indirect ELISA. Parasitol Res. 2011;109:1577-83. https://doi.org/10.1007/s00436-011-2414-4
Thiengo S, Fernandez M, Torres E, Coelho P, Lanfredi R. First record of a nematode Metastrongyloidea (Aelurostrongylus abstrusus larvae) in Achatina (Lissachatina) fulica (Mollusca, Achatinidae) in Brazil. J Invertebr Pathol. 2008;98:34-9. https://doi.org/10.1016/j.jip.2007.10.010
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621-30. https://doi.org/10.1016/S1473-3099(08)70229-9
Aziz NA, Daly E, Allen S, Rowson B, Greig C, Forman D, et al. Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural-urban gradient in two cities in the United Kingdom, using real time PCR. Parasit Vectors. 2016;2:56 https://doi.org/10.1186/s13071-016-1338-3
Some similar items:
- Anna Carolina Ratto-Tespestini, Paula Juliana Pérez-Chaparro, Giuseppe Alexandre Romito, Luciene Cristina Figueiredo, Marcelo Faveri, Hilana Paula Carillo, Priscila Larcher, Magda Feres, Comparison of independent and dependent culture methods for the detection of transient bacteremia in diabetic subjects with chronic periodontitis , Biomedica: Vol. 36 No. 1 (2016)
- Luis Solórzano-Alava, Francisco Sánchez-Amador, Talia Valverde, Angiostrongylus (Parastrongylus) cantonensis on intermediate and definitive hosts in Ecuador, 2014-2017 , Biomedica: Vol. 39 No. 2 (2019)
- Fernando Bolaños, Leonardo F. Jurado, Rina L. Luna-Tavera, Jaime M. Jiménez, Abdominal angiostrongyliasis, report of two cases and analysis of published reports from Colombia , Biomedica: Vol. 40 No. 2 (2020)
- Zulbey Rivero, Lisbeth Villareal, Ángela Bracho, Carem Prieto, Rafael Villalobos, Molecular identification of Entamoeba histolytica, E. dispar, and E. moshkovskii in children with diarrhea from Maracaibo, Venezuela , Biomedica: Vol. 41 No. Supl. 1 (2021): Mayo, Parasitología médica
- Lina M. Osorio-Cock, Sandra Catalina Jaramillo-Pulgarín, Alba P. Ferrín-Bastidas, Diana Y. Molina-Colorado, Óscar M. Gómez-Guzmán, Alejandra Zuluaga, Juan G. McEwen-Ochoa, Martha E. Urán-Jiménez, María del Pilar Jiménez-Alzate, Pseudoepitheliomatous hyperplasia: Squamous cell carcinoma versus oral paracoccidioidomycosis, a case from a dermatological perspective , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Fernando Almeida-Siva, Rodrigo Almeida-Paes , Lisandra Serra-Damasceno , Edwiges Motta-Santos , Luiz Claudio Ferreira, Leonardo Pereira-Quintella, Marcela de Faria Ferreira , Mauro de Medeiros-Muniz , Rosely M. Zancopé-Oliveira , The conventional diagnosis challenge: Real-time PCR and nested PCR correlation with the scoring system for individuals at high-risk of Pneumocystis jirovecii pneumonia , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























