Detection of Leishmania (V) guyanensis in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) collected from Pecari tajacu
Abstract
Introduction: Previous studies identified the presence of Leishmania infantum in Rhipicephalus sanguineus and indicated the possibility that it could transmit leishmaniasis to a variety of hosts.
Objective: To identify parasites of Leishmania (Viannia) spp. in ticks collected from wild animals in an endemic area for leishmaniasis.
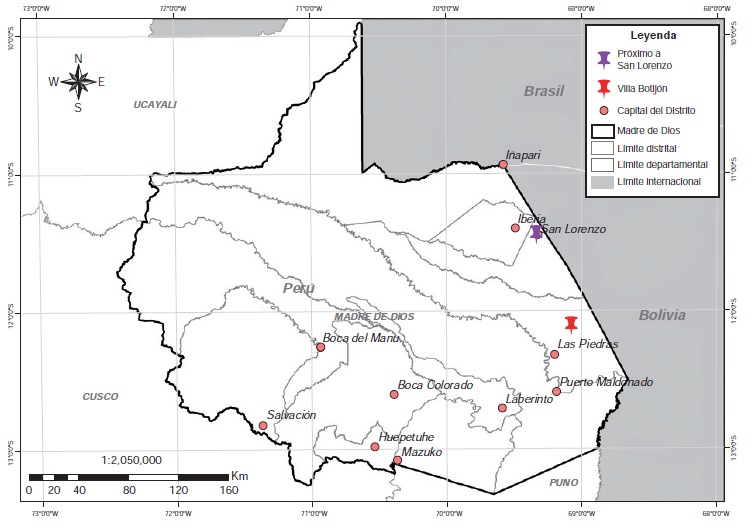
Materials and methods: We performed 81 individual DNA extractions from ticks collected from three Tapirus terrestris and three Pecari tajacu in Madre de Dios, Perú. Ticks were taxonomically identified and they were subsequently prepared to identify Leishmania (Viannia) spp. kDNA by PCR and the species of Leishmania by HRM-PCR.
Results: Leishmania (Viannia) kDNA was detected in three wild ticks of the species R. microplus, collected from a collard peccary (P. tajacu) hunted in the forests of Madre de Dios. The HRM-PCR showed that one of the positive samples had a kDNA curve compatible with L. (V) guyanensis.
Conclusion: The results showed the presence of L. (V) guyanensis DNA in R. microplus possibly acquired after biting a collarde peccary. Therefore, it is important to design future studies to clarify R. microplus involvement in the transmission of leishmaniasis.
Downloads
References
Dantas-Torres F. Canine leishmaniosis in South America. Parasit Vectors. 2009;2(Suppl.1):1-8. https://doi.org/10.1186/
-3305-2-S1-S1
Solano-Gallego L, Rossi L, Scroccaro A, Montarsi F, Caldin M, Furlanello T, et al. Detection of Leishmania ticks removed from dogs living in endemic areas of canine leishmaniosis. Parasit Vectors. 2012;5:98. https://doi.org/10.1186/1756-3305-5-98
Colombo FA, Odorizzi RM, Laurenti MD, Galati EA, Canavez F, Pereira-Chioccola VL. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res. 2011;2:267-74.
https://doi.org/10.1007/s00436-010-2247-6
Dantas-Torres F, Latrofa MS, Otranto D. Quantification of Leishmania infantum DNA in females, eggs, and larvae of Rhipicephalus sanguineus. Parasit Vectors. 2011;4:56. https://doi.org/10.1186/1756-3305-4-56
Dantas-Torres F. Ticks as vectors of Leishmania parasites. Trends Parasitol. 2011;27:155-9. https://doi.org/10.1016/j.pt.2010.12.006
Otranto D, Testini G, Dantas-Torres F, Latrofa MS, Vissotto de Paiva Diniz PP, de Caprariis D, et al. Diagnosis of canine vector-borne diseases in young dogs: A longitudinal study. J Clin Microbiol. 2010;48:3316-24. https://doi.org/10.1128/JCM.00379-10
Dantas-Torres F, Testini LV, de Paiva-Cavalcanti M, Figueredo LA, Stanneck D, Mencke N, et al. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol Res. 2010;106:857-60. https://doi.org/10.1007/s00436-010-1722-4
Sabogal S. Filogeografía y conservación genética del pecarí de collar, Pecari tajacu, en cuatro departamentos de Colombia (tesis). Bogotá: Universidad Nacional de Colombia; 2010.
Barros-Battesti D, Arzua M, Bechara H. Carrapatos de Importância Medico-Veterinaria da Região Neotropical: Um Guia Ilustrado para Identificação de Espécies. 10ma edição. Sao Paulo: Butantan Publicação; 2006. p. 223.
QIAGEN. Gentra®, Puregene® (QIAGEN Group), 2007-2010. Fecha de consulta: 9 de junio de 2017. Disponible en: https://
www.qiagen.com/us/shop/sample-technologies/dna/genomicdna/gentra-puregene-tissue-kit/#orderinginformation.
López M, Inga R, Cangalaya M, Echevarría J, Llanos-Cuentas A. Diagnosis the Leishmania using the polimerase chain reaction: A simplified procedure for field work. Am J Trop Med Hyg.1993;49:348-56. https://doi.org/10.4269/ajtmh.1993.49.348
Cabrera O, Munstermann L, Cárdenas R, Gutiérrez R, Ferro C. Definición de las condiciones de temperatura y almacenamiento adecuadas en la detección de ADN de Leishmania por PCR en flebotominos. Biomédica. 2002;22:296-302. https://doi.org/10.7705/biomedica.v22i3.1167
Labruna M, Romero M, Martins T, Tobler M, Ferrerira F.Ticks of the genus Amblyomma (Acari: Ixodidae) infesting tapirs (Tapirus terrestris) and peccaries (Tayassu pecari) in Perú. Syst Appl Acarol. 2010;15:109-12. https://doi.org/10.11158/saa.15.2.3
Otranto D, Dantas-Torres F. Canine and feline vector-borne diseases in Italy: Current situation and perspectives. Parasit Vectors. 2010;3:2. https://doi.org/10.1186/1756-3305-3-2
Rodríguez-Vivas R, Hodgkinson J, Trees A. Resistencia a los acaricidas en Rhipicephalus (Boophilus) microplus: situación actual y mecanismos de resistencia. Rev Mex Cienc Pecu. 2012;3:9-24.
Chen Z, Liu Q, Liu J, Xu B, Lv S, Xia S, et al. Tick-borne pathogens and associated co-infections in ticks collected
from domestic animals in central China. Parasit Vectors. 2014;7:237. https://doi.org/10.1186/1756-3305-7-237
Madder M, Adehan S, De Deken R, Adehan R, Lokossou R. New foci of Rhipicephalus microplus in West Africa. Exp Appl Acarol. 2012;56:385-90. https://doi.org/10.1007/s10493-012-9522-4
Guerrero F, Andreotti R, Bendele K, Cunha R, Miller R, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors. 2014;7:475. https://doi.org/1010.1186/s13071-014-0475-9
Goncalvez L, Filgueira K, Ahid S, Pereira J, Mendes do Vale A, Machado R, et al. Study on coinfecting vectorborne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet. 2014;23:407-12. https://doi.org/10.1590/S1984-29612014071
Silva de Morais R, Goncalves-de-Albuquerquea S, Pessoa e Silva R, Lemos P, Gaudêncio da Silva K, Brandão-Filho S, et al. Detection and quantification of Leishmania braziliensis in ectoparasites from dogs. Vet Parasitol. 2013;196:506-8. https://doi.org/10.1016/j.vetpar.2013.03.026
Campos J, Costa F. Participation of ticks in the infectious cycle of canine visceral leishmaniasis, in Teresina, Piauí, Brazil. Rev Inst Med Trop Sao Paulo. 2014;56:297-300. https://doi.org/10.1590/S0036-46652014000400005
Ceccarelli M, Galluzzi L, Migliazzo A, Magnani M. Detection and characterization of Leishmania (Leishmania) and Leishmania (Viannia) by SYBR green-based real-time PCR and high resolution melt analysis targeting kinetoplast minicircle DNA. PLoS One. 2014;9:e88845. https://doi.org/10.1371/journal.pone.0088845
Lopes E, Geraldo C, Marcili A, Silva R, Keid L, Oliverira T, et al. Performance of conventional PCRs based on primers directed to nuclear and mitochondrial genes for the detection and identification of Leishmania spp. Rev Inst Med Trop Sao Paulo. 2016;58:41. https://doi.org/10.1590/S1678-9946201658041
Some similar items:
- Diego Fernando Zea, Martín Prager, Roger Adrian Figueroa, María Consuelo Miranda, Mucosal complication of cutaneous leishmaniasis , Biomedica: Vol. 29 No. 1 (2009)
- Olga Lucía Cabrera, Laureano Mosquera, Erika Santamaría, Sand flies (Diptera: Psychodidae) of Guaviare Province, Colombia, with 4 new records for the country , Biomedica: Vol. 29 No. 1 (2009)
- Luis Alberto Cortés, Jhon James Fernández, Species of Lutzomyia involved in an urban focus of visceral and cutaneous leishmaniasis , Biomedica: Vol. 28 No. 3 (2008)
- Jazzmin Arrivillaga, Jaime Rodríguez, Milagros Oviedo, Preliminary evaluation of maggot (Diptera: Calliphoridae) therapy as a potential treatment for leishmaniasis ulcers , Biomedica: Vol. 28 No. 2 (2008)
- Rafael José Vivero, Maria Angélica Contreras-Gutiérrez, Eduar Elías Bejarano, Analysis of the primary and secondary structure of the mitochondrial serine transfer RNA in seven species of Lutzomyia , Biomedica: Vol. 27 No. 3 (2007)
- Guillermo Terán-Angel, Henk Schallig, Olga Zerpa, Vestalia Rodríguez, Marian Ulrich, Maira Cabrera, The direct agglutination test as an alternative method for the diagnosis of canine and human visceral leishmaniasis , Biomedica: Vol. 27 No. 3 (2007)
- Elsa Nieves, Neudo Buelvas, Maritza Rondón, Néstor González, The salivary glands of two sand fly vectors of Leishmania: Lutzomyia migonei (França) and Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) , Biomedica: Vol. 30 No. 3 (2010)
- Carlos Pérez, Yoanet Solías, Gerzaín Rodríguez, Diffuse cutaneous leishmaniasis in a patient with AIDS , Biomedica: Vol. 26 No. 4 (2006)
- María Angélica Contreras, Rafael José Vivero, Eduar Elías Bejarano, Lina María Carrillo, Iván Darío Vélez, New records of phlebotomine sand flies (Diptera: Psychodidae) near the Amoya River in Chaparral, Tolima , Biomedica: Vol. 32 No. 2 (2012)
- Sandra Uribe, Polymorphism in Populations of Lutzomyia longipalpis, Agent of Visceral Leishmaniasis, Suggests the Existence of a Species Complex , Biomedica: Vol. 19 No. 1 (1999)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























