Diagnostic accuracy of three technologies for the diagnosis of multi-drug resistant tuberculosis
Abstract
Introduction: Multi-drug resistant (MDR-TB) and extensively drug-resistant (XDR-TB) tuberculoses are a global public health problem. Their timely detection might reduce the burden of the disease and the economic impact on health systems worldwide.
Objective: To conduct a literature review of the diagnostic accuracy of three molecular tests to detect multi-drug resistant and extensively drug-resistant tuberculoses.
Materials and methods: A systematic literature review following Cochrane methodology was carried out to study the diagnostic accuracy of three molecular tests to detect MDR-TB and XDR-TB in previous studies among immunocompetent population. Articles indexed in Medline and Embase were reviewed starting in 2007. Diagnostic accuracy was reported by sensitivity, specificity, and positive and negative predictive values of each test.
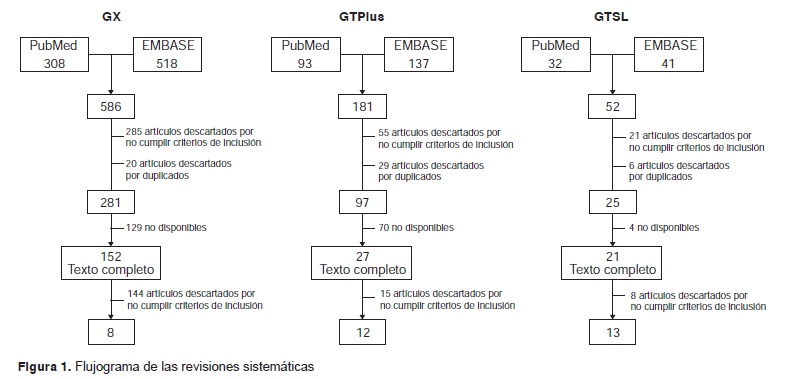
Results: In total, 8, 12 and 13 studies were included to assess the diagnostic accuracy of GeneXpert MTB/RIF®, GenoType MTBDRplus® and GenoType MTBDRsl®, respectively. The specificity of GeneXpert MTB/RIF® ranged between 91 and 100%, and its sensitivity between 33.3 and 100%. The sensitivity of GenoType® MTBDRplus® ranged between 88 and 100%. The sensitivity and specificity of GenoType MTBDRsl® to evaluate drug resistance ranged between 56 and 100% and 21 and 100%, respectively.
Conclusion: The three diagnostic tests evaluated have shown an adequate diagnostic accuracy to detect MDR and XDR tuberculoses.
Downloads
References
Organización Mundial de la Salud. Tuberculosis. Nota descriptiva No. 104. 2016. Fecha de consulta: 1 de marzo de 2016. Disponible en: http://www.who.int/mediacentre/factsheets/fs104/es/
Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane database Syst Rev. 2013;1:CD009593. https://doi.org/10.1002/14651858.CD009593.pub2
Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005-70. https://doi.org/10.1016/S0140-6736(14)60844-8
Organización Mundial de la Salud. Temas de salud. Tuberculosis 2015. Fecha de consulta: 17 de febrero de 2015. Disponible en: http://www.who.int/topics/tuberculosis/es/
Lynch JB. Multidrug-resistant tuberculosis. Med Clin North Am. 2013;97:553-79. https://doi.org/10.1016/j.mcna.2013.03.012
Moonan PK, Teeter LD, Salcedo K, Ghosh S, Ahuja SD, Flood J, et al. Transmission of multidrug-resistant tuberculosis in the USA: A cross-sectional study. Lancet Infect Dis. 2013;13:777-84. https://doi.org/10.1016/S1473-3099(13)70128-2
Caminero JA. Multidrug-resistant tuberculosis: Epidemiology, risk factors and case finding. Int J Tuberc Lung Dis. 2010;14:382-90.
Mishra R, Shukla P, Huang W, Hu N. Gene mutations in Mycobacterium tuberculosis: Multidrug-resistant TB as an emerging global public health crisis. Tuberculosis. 2015;95:1-5. https://doi.org/10.1016/j.tube.2014.08.012
World Health Organization. Global tuberculosis control 2008, surveillance, planning, financing. WHO/HTM/TB/2008.393. Geneva: WHO; 2008.
Xu B, Hu Y, Zhao Q, Wang W, Jiang W, Zhao G. Molecular epidemiology of TB –Its impact on multidrugresistant tuberculosis control in China. Int J Mycobacteriol. 2015;4:134. https://doi.org/10.1016/j.ijmyco.2014.09.003
Migliori GB, Richardson MD, Sotgiu G, Lange C. Multidrugresistant and extensively drug-resistant tuberculosis in the West Europe and United States: Epidemiology, surveillance, and control. Clin Chest Med. 2009;30:637-65. https://doi.org/10.1016/j.ccm.2009.08.015
World Health Organization. Global tuberculosis report 2015. WHO/HTM/TB/2015.22. Geneva: WHO; 2015.
Organización Mundial de la Salud. Estrategia “Alto a la tuberculosis”, 2015. Fecha de consulta: 19 de febrero de 2015. Disponible en: http://www.who.int/tb/strategy/stop_tb_strategy/es/
Bwanga F, Hoffner S, Haile M, Joloba ML, Blumberg H, Burman W, et al. Direct susceptibility testing for multi drug resistant tuberculosis: A meta-analysis. BMC Infect Dis. 2009;9:67. https://doi.org/10.1186/1471-2334-9-67
World Health Organization. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). Geneva: WHO; 2008.
Hain Lifescience. GenoType MTBDRplus. Fecha de consulta: 8 de noviembre de 2016. Disponible en: http://www.hainlifescience.de/en/products/microbiology/mycobacteria/tuberculosis/genotype-mtbdrplus.html
Asencios L, Galarza M, Quispe N, Vásquez L, Leo E, Valencia E, et al. Molecular test Genotype® MTBDRplus, an alternative to rapid detection of multidrug resistance tuberculosis. Rev Perú Med Exp Salud Pública. 2012;29:92-8. https://doi.org/10.1590/S1726-46342012000100014
Raizada N, Sachdeva KS, Chauhan DS, Malhotra B, Reddy K, Dave P V., et al. A multi-site validation in India of the line probe assay for the rapid diagnosis of multi-drug resistant tuberculosis directly from sputum specimens. PLoS One. 2014;9:e88626. https://doi.org/10.1371/journal.pone.0088626
World Health Organization. The use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs. 2013. Fecha de consulta: 8 de noviembre de 2016. Disponible en: http://apps.who.int/iris/bitstream/10665/78099/1/WHO_HTM_TB_2013.01.eng.pdf?ua=1
Felkel M, Exner R, Schleucher R, Lay H, Autenrieth IB, Kempf VA, et al. Evaluation of Mycobacterium tuberculosis drug susceptibility in clinical specimens from Nigeria using genotype MTBDRplus and MTBDRsl assays. Eur J Microbiol Immunol. 2013;3:252-7. https://doi.org/10.1556/EuJMI.3.2013.4.3
World Health Organization. WHO endorses new rapid tuberculosis test. Fecha de consulta: 8 de noviembre de 2016. Disponible en: http://www.who.int/mediacentre/news/releases/2010/tb_test_20101208/en/
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-15. https://doi.org/10.1056/NEJMoa0907847
World Health Organization. Implementing tuberculosis diagnostics: A policy framework. 2015. Fecha de consulta: 31 de octubre de 2016. Disponible en: http://www.who.int/tb/publications/implementing_TB_diagnostics/en/
Ministerio de Salud y Protección Social. ¿Qué es tuberculosis (TB)? Bogotá D.C. 2016. Fecha de consulta: 1 de enero de 2016. Disponible en: https://www.minsalud.gov.co/salud/publica/PET/Paginas/Tuberculosis.aspx
Garzón MC, Angée DY, Llerena C, Orjuela DL, Victoria JE. Vigilancia de la resistencia del Mycobacterium tuberculosis a los fármacos antituberculosos, Colombia 2004-2005. Biomédica. 2008;28:319-26. https://doi.org/10.7705/biomedica.v28i3.71
Instituto Nacional de Salud. Informe de actividades realizadas por la Red Nacional de Laboratorios para la vigilancia de la resistencia de Mycobacterium tuberculosis a los fármacos antituberculosos, Colombia, 2013. Fecha de consulta: 1 de enero de 2016. Disponible en: http://www.ins.gov.co/lineas-de-accion/Red-Nacional-Laboratorios/Documentacin%20Micobacterias/INFORME%20DE%20LA%20VIGILANCIA%20DE%20LA%20RESISTENCIA%20DE%20MYCOBACTERIUM%20TUBERCULOSIS%20A % 2 0 L O S % 2 0 F % C 3 % 8 1 R M A C O S % 2 0ANTITUBERCULOSOS%202013.pdf
Bunsow E, Ruiz-Serrano MJ, Roa PL, Kestler M, Viedma DG, Bouza E. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. J Infect. 2014;68:338-43. https://doi.org/10.1016/j.jinf.2013.11.012
Banco Mundial. Datos. Clasificación por países. Fecha de consulta: 22 de noviembre de 2015. Disponible en: http://datos.bancomundial.org/pais
Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet. 2011;377:1495-505. https://doi.org/10.1016/S0140-6736(11)60438-8
Bowles EC, Freyée B, Van Ingen J, Mulder B, Boeree MJ, Van Soolingen D. Xpert MTB/RIF®, a novel automated polymerase chain reaction–based tool for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2011;15:988-9. https://doi.org/10.5588/ijtld.10.0574
Huh HJ, Jeong B-H, Jeon K, Koh W-J, Ki C-S, Lee NY. Performance evaluation of the Xpert MTB/RIF assay according to its clinical application. BMC Infect Dis. 2014;14:589. https://doi.org/10.1186/s12879-014-0589-x
Myneedu VP, Behera D, Verma AK, Bhalla M, Singh N, Arora J, et al. Xpert® MTB/RIF assay for tuberculosis diagnosis: Evaluation in an Indian setting. Int J Tuberc Lung Dis. 2014;18:958-60. https://doi.org/10.5588/ijtld.13.0328
Ou X, Xia H, Li Q, Pang Y, Wang S, Zhao B, et al. A feasibility study of the Xpert MTB/RIF test at the peripheral level laboratory in China. Int J Infect Dis. 2015;31:41-6. https://doi.org/10.1016/j.ijid.2014.09.011
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593. https://doi.org/10.1002/14651858.CD009593.pub3
Williamson DA, Basu I, Bower J, Freeman JT, Henderson G, Roberts SA. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2012;74:207-9. https://doi.org/10.1016/j.diagmicrobio.2012.06.013
Hanif SN, Eldeen HS, Ahmad S, Mokaddas E. GeneXpert® MTB/RIF for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary samples. Int J Tuberc Lung Dis. 2011;15:1274-5. https://doi.org/10.5588/ijtld.11.0394
Ioannidis P, Papaventsis D, Karabela S, Nikolaou S, Panagi M, Raftopoulou E, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011;49:3068-70. https://doi.org/10.1128/JCM.00718-11
Kurbatova EV, Kaminski DA, Erokhin VV, Volchenkov GV, Andreevskaya SN, Chernousova LN, et al. Performance of Cepheid® Xpert MTB/RIF® and TB-Biochip® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2013;32:735-43. https://doi.org/10.1007/s10096-012-1798-0
Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and non-respiratory samples. Int J Tuberc Lung Dis. 2011;15:553-5. https://doi.org/10.5588/ijtld.10.0497
Marlowe EM, Novak-Weekley SM, Cumpio J, Sharp SE, Momeny MA, Babst A, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:1621-3. https://doi.org/10.1128/JCM.02214-10
Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49:3458-62. https://doi.org/10.1128/JCM.05212-11
Safianowska A, Walkiewicz R, Nejman-Gryz P, Grubek-Jaworska H. Two selected commercially based nucleic acid amplification tests for the diagnosis of tuberculosis. Pneumonol Alergol Pol. 2011;80:6-12.
Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium Tuberculosis Direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011;49:3659-62. https://doi.org/10.1128/JCM.00211-11
Hanrahan CF, Selibas K, Deery CB, Dansey H, Clouse K, Bassett J, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8:e65421. https://doi.org/10.1371/journal.pone.0065421
Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138-41. https://doi.org/10.1128/JCM.05434-11
Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay—a clinical validation study. PLoS One. 2011;6:e20458. https://doi.org/10.1371/journal.pone.0020458
Ajbani K, Nikam C, Kazi M, Gray C, Boehme C, Balan K, et al. Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments. PLoS One. 2012;7:e49433. https://doi.org/10.1371/journal.pone.0049433
Barnard M, Warren R, Van Pittius NG, van Helden P, Bosman M, Streicher E, et al. Genotype MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am J Respir Crit Care Med. 2012;186:1298-305. https://doi.org/10.1164/rccm.201205-0960OC
Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683-9. https://doi.org/10.1128/JCM.01947-09
Ferro BE, García PK, Nieto LM, van Soolingen D. Predictive value of molecular drug resistance testing of Mycobacterium tuberculosis isolates in Valle del Cauca, Colombia. J Clin Microbiol. 2013;51:2220-4. https://doi.org/10.1128/JCM.00429-13
Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2009;47:1767-72. https://doi.org/10.1128/JCM.00081-09
Huang W-L, Chi T-L, Wu M-H, Jou R. Performance assessment of the GenoType MTBDRsl test and DNA sequencing for detection of second-line and ethambutol drug resistance among patients infected with multidrugresistant Mycobacterium tuberculosis. J Clin Microbiol.
;49:2502-8. https://doi.org/10.1128/JCM.00197-11
Ignatyeva O, Kontsevaya I, Kovalyov A, Balabanova Y, Nikolayevskyy V, Toit K, et al. Detection of resistance to second-line antituberculosis drugs by use of the genotype MTBDRsl assay: A multicenter evaluation and feasibility study. J Clin Microbiol. 2012;50:1593-7. https://doi.org/10.1128/JCM.00039-12
Jin J, Shen Y, Fan X, Diao N, Wang F, Wang S, et al. Underestimation of the resistance of Mycobacterium tuberculosis to second-line drugs by the new GenoType MTBDRsl test. J Mol Diagnostics. 2013;15:44-50. https://doi.org/10.1016/j.jmoldx.2012.08.004
Kontsevaya I, Mironova S, Nikolayevskyy V, Balabanova Y, Mitchell S, Drobniewski F. Evaluation of two molecular assays for rapid detection of Mycobacterium tuberculosis resistance to fluoroquinolones in high-tuberculosis andmultidrug-resistance settings. J Clin Microbiol. 2011;49:2832-7. https://doi.org/10.1128/JCM.01889-10
Lacoma A, García-Sierra N, Prat C, Maldonado J, Ruiz-Manzano J, Haba L, et al. GenoType MTBDRsl for molecular detection of second-line-drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2012;50:30-6. https://doi.org/10.1128/JCM.05274-11
López-Roa P, Ruiz-Serrano MJ, Alcalá L, Ráez NG-E, de Viedma DG, Bouza E. Susceptibility testing to secondline drugs and ethambutol by GenoType MTBDRsl and Bactec MGIT 960 comparing with agar proportion method. Tuberculosis. 2012;92:417-21. https://doi.org/10.1016/j.tube.2012.05.005
Said HM, Kock MM, Ismail NA, Baba K, Omar SV, Osman AG, et al. Evaluation of the GenoType® MTBDRsl assay for susceptibility testing of second-line anti-tuberculosis drugs. Int J Tuberc Lung Dis. 2012;16:104-9. https://doi.org/10.5588/ijtld.10.0600
Tukvadze N, Bablishvili N, Apsindzelashvili R, Blumberg HM, Kempker RR. Performance of the MTBDRsl assay in Georgia. Int J Tuberc Lung Dis. 2014;18:233-9. https://doi.org/10.5588/ijtld.13.0468
Chen C, Kong W, Zhu L, Zhou Y, Peng H, Shao Y, et al. Evaluation of the GenoType® MTBDRplus line probe assay on sputum-positive samples in routine settings in China. Int J Tuberc Lung Dis. 2014;18:1034-9. https://doi.org/10.5588/ijtld.13.0857
de Abreu Maschmann R, Spies FS, de Souza Nunes L, Ribeiro AW, Machado TRM, Zaha A, et al. Performance of the GenoType MTBDRplus assay directly on sputum specimens from Brazilian patients with tuberculosis treatment failure or relapse. J Clin Microbiol. 2013;51:1606-8. https://doi.org/10.1128/JCM.00364-13
Dorman SE, Chihota VN, Lewis JJ, van der Meulen M, Mathema B, Beylis N, et al.Genotype MTBDRplus for direct detection of Mycobacterium tuberculosis and drug resistance in strains from gold miners in South Africa. J Clin Microbiol. 2012;50:1189-94. https://doi.org/10.1128/JCM.05723-11
Jin J, Zhang Y, Fan X, Diao N, Shao L, Wang F, et al. Evaluation of the GenoType® MTBDRplus assay and identification of a rare mutation for improving MDR-TB detection. Int J Tuberc Lung Dis. 2012;16:521-6. https://doi.org/10.5588/ijtld.11.0269
Causse M, Ruiz P, Gutiérrez JB, Zerolo J, Casal M. Evaluation of new GenoType® MTBDRplus for detection of resistance in cultures and direct specimens of Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2008;12:1456-60.
Chryssanthou E, Ängeby K. The GenoType® MTBDRplus assay for detection of drug resistance in Mycobacterium tuberculosis in Sweden. APMIS. 2012;120:405-9. https://doi.org/10.1111/j.1600-0463.2011.02845.x
Lyu J, Kim MN, Song JW, Choi CM, Oh YM, Lee SD, et al. GenoType® MTBDRplus assay detection of drug-resistant tuberculosis in routine practice in Korea. Int J Tuberc Lung Dis. 2013;17:120-4. https://doi.org/10.5588/ijtld.12.0197
Buyankhishig B, Oyuntuya T, Tserelmaa B, Sarantuya J, Lucero MG, Mitarai S. Rapid molecular testing for multi-resistant tuberculosis in Mongolia: A diagnostic accuracy study. Int J Mycobacteriol. 2012;1:40-4. https://doi.org/10.1016/j.ijmyco.2012.01.007
Farooqi JQ, Khan E, Alam SMZ, Ali A, Hasan Z, Hasan R. Line probe assay for detection of rifampicin and isoniazid resistant tuberculosis in Pakistan. J Pak Med Assoc. 2012;62:767-72.
Huyen MN, Tiemersma EW, Lan NT, Cobelens FG, Dung NH, Sy DN, et al. Validation of the GenoType® MTBDRplus assay for diagnosis of multidrug resistant tuberculosis in South Vietnam. BMC Infect Dis. 2010;10:149. https://doi.org/10.1186/1471-2334-10-149
Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, et al. Reducing the global burden of tuberculosis: The contribution of improved diagnostics. Nature. 2006;444:49-57. https://doi.org/10.1038/nature05446
World Health Organization. Strategic and Technical Advisory Group for Tuberculosis: Report of 10th meeting. Geneva: WHO; 2010.
Lin H-H, Langley I, Mwenda R, Doulla B, Egwaga S, Millington KA, et al. A modelling framework to support the selection and implementation of new tuberculosis diagnostic tools [State of the art series. Operational research. Number 8 in the series]. Int J Tuberc Lung Dis. 2011;15:996-1004.
https://doi.org/10.5588/ijtld.11.0062
Altman DG, Bossuyt PM. Estudios de precisión diagnóstica (STARD) y pronóstica (REMARK). Med Clin (Barc). 2005;125: 49-55. https://doi.org/10.1016/S0025-7753(05)72210-7
Some similar items:
- Claudia Llerena, Angie Zabaleta, Angélica Valbuena, Martha Murcia, Prevalence of Mycobacterium tuberculosis resistance to quinolones and injectables in Colombia, 2012-2013 , Biomedica: Vol. 37 No. 1 (2017)
- Johana Rueda, Teresa Realpe, Gloria Mejía, Elsa Zapata, Jaime Robledo, GenoType MTBDRplus 1.0® for the detection of cross-resistance between isoniazide and ethionamide in isolates of multidrug-resistant Mycobacterium tuberculosis , Biomedica: Vol. 35 No. 4 (2015)
- Dihadenys Lemus, Miguel Echemendía, Raúl Díaz, Alina Llop, María Josefa Llanes, Surveillance of antituberculosis-drug resistance in Cuba, 2010-2011 , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Claudia Llerena, Santiago Elías Fadul, María Consuelo Garzón, Graciela Mejía, Dora Leticia Orjuela, Luz Mary García, Hilda Beatriz Álvarez, Fernando Javier Ruiz, Drug-resistant Mycobacterium tuberculosis in children under 15 years , Biomedica: Vol. 30 No. 3 (2010)
- María Consuelo Garzón, Dailyn Yorledy Angée, Claudia Llerena, Dora Leticia Orjuela, Jorge Ernesto Victoria, Surveillance of Mycobacterium tuberculosis resistance to antituberculosis drugs , Biomedica: Vol. 28 No. 3 (2008)
- Astrid Elena Montoya, José Menco, Natalia Osorio, Maria Alejandra Zuluaga, Juliana Duque, Giovanny Torres, Marcos Restrepo, Concordance between thick blood smear, immunochromatography and polymerase chain reaction for malaria diagnosis , Biomedica: Vol. 28 No. 2 (2008)
- Gloria Mercedes Puerto , Claudia Marcela Castro , Vivian Vanesa Rubio , Santiago Fadul, Fernando Montes , Drug-resistant tuberculosis in Colombia, 2013-2018: Case-control study , Biomedica: Vol. 43 No. 4 (2023)
- Ruth Aralí Martínez-Vega, Fredi Alexander Díaz Quijano, Carolina Coronel Ruiz, Sergio Yebrail Gómez, Luis Ángel Villar Centeno, Evaluation of PANBIO rapid immunochromatographic cassette for dengue diagnosis in a Colombian endemic area , Biomedica: Vol. 29 No. 4 (2009)
- Diego Chaves, Andrea Sandoval, Luis Rodríguez, Juan C. García, Silvia Restrepo, María Mercedes Zambrano, Comparative analysis of six Mycobacterium tuberculosis complex genomes , Biomedica: Vol. 30 No. 1 (2010)
- Luis Miguel Sosa, Luz Libia Cala, Julio César Mantilla, Congenital tuberculosis associated with maternal disseminated miliary tuberculosis , Biomedica: Vol. 27 No. 4 (2007)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























