Developmental stages and viability of Toxocara canis eggs outside the host
Abstract
Introduction: Toxocariasis is a soil-transmitted zoonotic disease caused mainly by ingestion of larvated eggs of Toxocara canis.
Objectives: To study the morphology of the intraovular developmental stages of Toxocara canis in culture, characterize non-viable eggs and the sequences of larval molting and compare the viability of eggs at the early stages of division and at reaching full maturation.
Material and methods: Observation of developing embryos and characterization of non-viable eggs were done using light microscope. The proportions of viable eggs during embryonation were compared to the proportions of viable mature eggs.
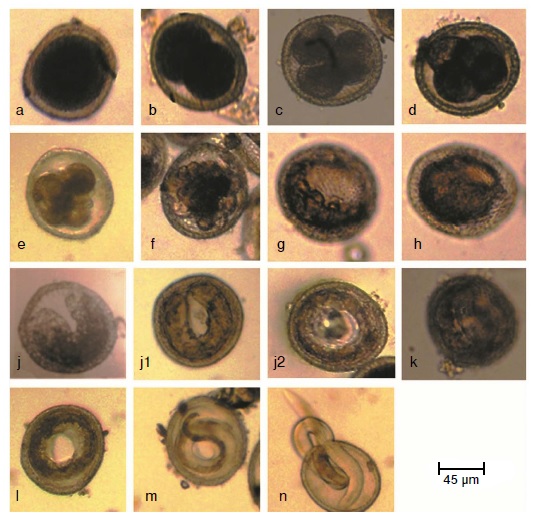
Results: Cell division commenced after 24 hours of cultivation. Early stages were found to be present over a period of 3-5 days. The developmental stages identified were eggs with: One cell, two cells, three cells, four cells, early morula, late morula, blastula, gastrula, tadpole, pre-larva, first, second and third stage larva. Two larval molts occurred. Non-viable eggs had degenerated cytoplasm, thin or collapsed shell and the larvae did not move after exposure to light. No significant differences were found between the proportions of viable eggs from day five to day 21 as compared to viability of fully mature eggs (30 days).
Conclusion: Developing embryos in the environment may be considered as a potential threat to the public health. The precise identification of developmental stages and the clear differentiation of viable and non-viable eggs can help in determining an accurate baseline rate of development that could be used in studies of ovicidal compounds.
Downloads
References
Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182-8. https://doi.org/10.1016/j.pt.2009.01.006
Moreira GM, Telmo P de L, Mendonça M, Moreira AN, McBride AJ, Scaini CJ, et al. Human toxocariasis: Current advances in diagnostics, treatment, and interventions. Trends Parasitol. 2014;30:456-64. https://doi.10.1016/j.pt.2014.07.003
Taira K, Saeed I, Permin A, Kapel CM. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol. 2004;121:115-24. https://doi.org/10.1016/j.vetpar.2004.01.018
Macpherson CN. The epidemiology and public health importance of toxocariasis: A zoonosis of global importance. Int J Parasitol. 2013;43:999-1008. https://doi.org/10.1016/j.ijpara.2013.07.004
Fan CK, Liao CW, Cheng YC. Factors affecting disease manifestation of toxocarosis in humans: Genetics and environment. Vet Parasitol. 2013;193:342-52. https://doi.org/10.1016/j.vetpar.2012.12.030
Eid MM, El-Kowrany SI, Othman AA, El Gendy DI, Saied EM. Immunopathological changes in the brain of immunosuppressed mice experimentally infected with Toxocara canis. Korean J Parasitol. 2015;53:51-8. https://doi.org/10.3347/kjp.2015.53.1
Azam D, Ukpai OM, Said A, Abd-Allah GA, Morgan ER. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol Res. 2012;110:649-56.
https://doi.org/10.1007/s00436-011-2536-8
Zdybel J, Cencek T, Karamon J, Kłapeć T. Effectiveness of selected stages of wastewater treatment in elimination of eggs of intestinal parasites. Bull Vet Inst Pulawy 2015;59:51-7. https://doi.org/10.1515/bvip-2015-0008
Traversa D, Frangipane di Regalbono A, Di Cesare A, La Torre F, Drake J, Pietrobelli M. Environmental contamination by canine geohelminths. Parasit Vectors. 2014;7:67. https://doi.org/10.1186/1756-3305-7-67
Kirchheimer R, Jacobs DE. Toxocara species egg contamination of soil from children’s play areas in southern England. Vet Rec. 2008;163:394-5. https://doi.org/10.1136/vr.163.13.394
Fitzgerald R, Ashley RF. Differential survival of Ascaris ova in wastewater sludge. J Water Pollut Control Fed. 1977;49:1722-4.
Morrondo P, Díez-Morrondo C, Pedreira J, Díez-Baños N, Sánchez-Andrade R, Paz-Silva A, et al.Toxocara canis larvae viability after disinfectant-exposition. Parasitol Res. 2006;99:558-61. https://doi.org/10.1007/s00436-006-0200-5
Dąbrowska J, Zdybel J, Karamon J, Kochanowski M, Stojecki K, Cencek T, et al. Assessment of viability of the nematode eggs (Ascaris, Toxocara, Trichuris) in sewage sludge with the use of LIVE/DEAD Bacterial Viability Kit. Ann Agric Environ Med. 2014;21:35-41.
Ayres RM, Mara DD. Analysis of wastewater for use in agriculture: A laboratory manual of parasitological and bacteriological techniques. Geneva: WHO; 1996. p. 1-35.
United States Environmental Protection Agency (USEPA). Control of pathogens and vector attraction in sewage sludge. Accessed: July 1st, 2003. Available from: https://www.epa.gov/sites/production/files/2015-04/documents/control_of_pathogens_and_vector_attraction_in_sewage_sludge_july_2003.pdf
Cruz LM, Allanson M, Kwa B, Azizan A, Izurieta R. Morphological changes of Ascaris spp. eggs during their development outside the host. J Parasitol. 2012;98:63-8. https://doi.org/10.1645/GE-2821.1
Onorato AR. The effects of temperature and humidity on the ova of Toxocara canis and Trichuris vulpis. Am J Hyg. 1932;16:266-87.
Brunaská M, Dubinský P, Reiterová K. Toxocara canis: Ultrastructural aspects of larval moulting in the maturing eggs. Int J Parasitol. 1995;25:683-90. https://doi.org/10.1016/0020-7519(94)00183-O
Gamboa MI. Effects of temperature and humidity on the development of eggs of Toxocara canis under laboratory conditions. J Helminthol. 2005;79:327-31. https://doi.org/10.1079/JOH2005287
Nichols RL. The etiology of visceral larva migrans. I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol. 1956;42:349-62.
De Savigny DH. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol. 1975;61:781-2.
El Naga IF. Toxocara canis: Determination of the origin of antigenic materials released from infective larvae. J Egypt Soc Parasitol. 2000;30:669-78.
Black MI, Scarpino PV, O’Donnell CJ, Meyer KB, Jones JV, Kaneshiro ES. Survival rates of parasite eggs in sludge during aerobic and anaerobic digestion. Appl Environ Microbiol. 1982;44:1138-43.
Johnson PW, Dixon R, Ross AD. An in-vitro test for assessing the viability of Ascaris suum eggs exposed to various sewage treatment processes. Int J Parasitol. 1998;28:627-33. https://doi.org/10.1016/S0020-7519(97)00210-5
O’Lorcain P. The effects of freezing on the viability of Toxocara canis and T. cati embryonated eggs. J Helminthol. 1995;69:169-71.
Camparoto ML, Fulan B, Colli CM, Paludo ML, Falavigna-Guilherme AL, Fernandez MA. Initial stage of development and migratory behavior of Toxocara canis larvae in BALB/c mouse experimental model. Genet Mol Res. 2008;7:444-50.
Prociv P. Intraovular development and moulting of Toxocara pteropodis. Int J Parasitol. 1989;19:749-55. https://doi.org/10.1016/0020-7519(89)90062-3
Foor WE. Ultrastructural aspects of oocyte development and shell formation in Ascaris lumbricoides. J Parasitol. 1967;53:1245-61.
Uhlikova M, Hubner J. A study on the morphology of early larval stages of Toxocara cati (Schrank, 1788). Folia Parasitol (Praha). 1982;29:165-6.
Prociv P. Observations on the morphology of Toxocara pteropodis eggs. J Helminthol. 1990;164:271-7.
Dziekońska-Rynko J, Rokicki J. Life cycle of the nematode Contracaecum rudolphii Hartwig, 1964 (sensu lato) from northern Poland under laboratory conditions. Helminthologia. 2007;44:95-102. https://doi.org/10.2478/s11687-007-0013-9
Geenen PL, Bresciani J, Boes J, Pedersen A, Eriksen L, Fagerholm HP, et al. The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. J Parasitol. 1999;85:616-22.
Overgaauw PA. Aspects of Toxocara epidemiology: Toxocarosis in dogs and cats. Crit Rev Microbiol. 1997;23:233-51. http://dx.doi.org/10.3109/10408419709115138
Kirchgässner M, Schmahl G, Al-Quraishy S, Ghaffar FA, Mehlhorn H. What are the infectious larvae in Ascaris suum and Trichuris muris? Parasitol Res. 2008;103:603-7. https://doi.org/10.1007/s00436-008-1018-0
Ooi HK, Lin CL, Wang JS. Effect of ozone treatment on Toxocara canis eggs. J. Vet Med Sci. 1998;60:169-73. https://doi.org/10.1292/jvms.60.169
Wharton D. Nematode egg-shells. Parasitology. 1980;81:447-63.
Mazurkiewicz-Zapałowicz K, Jaborowska-Jarmoluk M, Kołodziejczyk L, Kuźna-Grygiel W. Comparison of the effect of the chosenspecies of saprotrophic fungi on the development of Toxocaracanis and Ascaris suum eggs. Ann Parasitol. 2014;60:215-20.
Oksanen A, Eriksen L, Roepstorff A, Ilsøe B, Nansen P, Lind P. Embryonation and infectivity of Ascaris suum eggs: A comparison of eggs collected from worm uteri with eggs isolated from pig faeces. Acta Vet Scand. 1990;31:393-8.
Rahimian S, Gaulyb M, Das G. Embryonation ability of Ascaridia galli eggs isolated from worm uteri or host faeces. Vet Parasitol. 2016;215:29-34. https://doi.org/10.1016/j.vetpar.2015.10.026
Some similar items:
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Gustavo Pradilla, Julio César Mantilla, Reynaldo Badillo, Human rabies encephalitis by a vampire bat bite in an urban area of Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Luz Elena Velásquez, Catalina Gómez, Erika Valencia, Laura Salazar, Eudoro Casas, Paragonimosis in the peri-urban zone of Medellín, Antioquia , Biomedica: Vol. 28 No. 3 (2008)
- Jazzmin Arrivillaga, Jaime Rodríguez, Milagros Oviedo, Preliminary evaluation of maggot (Diptera: Calliphoridae) therapy as a potential treatment for leishmaniasis ulcers , Biomedica: Vol. 28 No. 2 (2008)
- Hollman Miller, Gerzaín Rodríguez, Tungiasis in native Amerindians in Vaupés province: epidemiology, clinical aspects, treatment, and prevention , Biomedica: Vol. 30 No. 2 (2010)
- Richard C. Pacheco, Mauricio C. Horta, Jonas Moraes-Filho, Alexandre C. Ataliba, Adriano Pinter, Marcelo B. Labruna, Rickettsial infection in capybaras (Hydrochoerus hydrochaeris from São Paulo, Brazil: serological evidence for infection by Rickettsia bellii and Rickettsia parkeri , Biomedica: Vol. 27 No. 3 (2007)
- Jessika Valderrama, Ingrid García, Germán Figueroa, Edilberto Rico, Juliana Sanabria, Nicolás Rocha, Edgar Parra, Cecilia Saad, Andrés Páez, Outbreaks of human rabies transmitted by vampire bats in Alto Baudó and Bajo Baudó municipalities, department of Chocó, Colombia, 2004-2005 , Biomedica: Vol. 26 No. 3 (2006)
- Esteban Arroyave, Andres Felipe Londoño, Juan Carlos Quintero, Piedad Agudelo-Florez, Margarita Arboleda, Francisco J. Díaz, Juan D. Rodas, Etiology and epidemiological characterization of non-malarial febrile syndrome in three municipalities of Urabá (Antioquia), Colombia , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Marylin Hidalgo, Alvaro A. Faccini-Martínez, Gustavo Valbuena, Tick-borne rickettsioses in the Americas: clinical and epidemiological advances, and diagnostic challenges , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Álvaro A. Faccini-Martínez, Hugo A. Sotomayor, Historical review of the plague in South America: a little-known disease in Colombia , Biomedica: Vol. 33 No. 1 (2013)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























