Neurogenesis and gliogenesis modulation in cerebral ischemia by CDK5 RNAi-based therapy
Abstract
Introduction: Cerebral ischemia is the third cause of death risk in Colombia and the first cause of physical disability worldwide. Different studies on the silencing of the cyclin-dependent kinase 5 (CDK5) have shown that reducing its activity is beneficial in ischemic contexts. However, its effect on neural cell production after cerebral ischemia has not been well studied yet.
Objective: To evaluate CDK5 silencing on the production of neurons and astrocytes after a focal cerebral ischemia in rats.
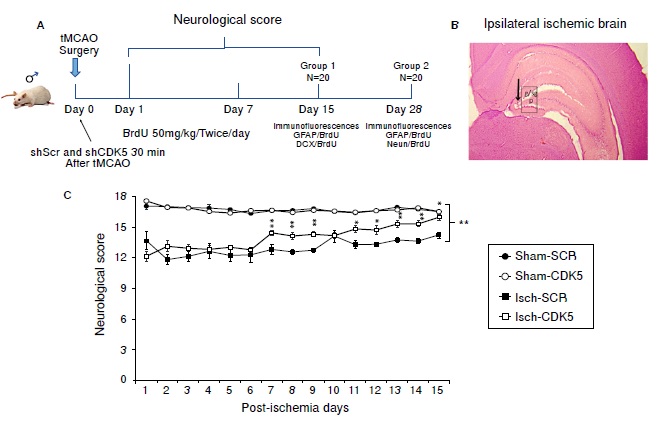
Materials and methods: We used 40 eight-week-old male Wistar rats. Both sham and ischemia groups were transduced at CA1 hippocampal region with an adeno-associated viral vector using a noninterfering (shSCRmiR) and an interfering sequence for CDK5 (shCDK5miR). We injected 50 mg/kg of bromodeoxyuridine intraperitoneally from hour 24 to day 7 post-ischemia. We assessed the neurological abilities during the next 15 days and we measured the immunoreactivity of bromodeoxyuridine (BrdU),
doublecortin (DCX), NeuN, and glial fibrillary acid protein (GFAP) from day 15 to day 30 post-ischemia.
Results: Our findings showed that CDK5miR-treated ischemic animals improved their neurological score and presented increased BrdU+ cells 15 days after ischemia, which correlated with higher DCX and lower GFAP fluorescence intensities, and, although mature neurons populations did not change, GFAP immunoreactivity was still significantly reduced at 30 days post-ischemia in comparison with untreated ischemic groups.
Conclusion: CDK5miR therapy generated the neurological recovery of ischemic rats associated with the induction of immature neurons proliferation and the reduction of GFAP reactivity at short and longterm post-ischemia.
Downloads
References
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399-415. https://doi.org/10.1038/nrn1106
Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2003;153(Suppl.1):S396-405. https://doi.org/10.1038/sj.bjp.0707626
Murphy TH, Corbett C. Plasticity during stroke recovery: From synapse to behavior. Nat Rev Neurosci. 2009;10:861-72. https://doi.org/10.1038/nrn2735
Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34-66. https://doi.org/10.1016/j.brainresrev.2006.11.003
Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke. 1995;26:2219-21. https://doi.org/10.1161/01.STR.26.12.2219
Posada-Duque RA, Barreto GE, Cardona-Gómez GP. Protection after stroke: Cellular effectors of neurovascular unit integrity. Front Cell Neurosci. 2014;8:231. https://doi.org/10.3389/fncel.2014.00231
Meyer DA, Torres-Altoro MI, Tan Z, Tozzi A, Di Filippo M, DiNapoli V, et al. Ischemic stroke injury is mediated by aberrant Cdk5. J Neurosci. 2014;34,8259-67. https://doi.org/10.1523/JNEUROSCI.4368-13.2014
Chang KH, de Pablo Y, Lee HP, Lee HG, Smith MA, Shah K. Cdk5 is a major regulator of p38 cascade: Relevance to neurotoxicity in Alzheimer’s disease. J Neurochem. 2010;113:1221-9. https://doi.org/10.1111/j.1471-4159.2010.06687.x
Lopes JP, Agostinho P. Cdk5: Multitasking between physiological and pathological conditions. Prog Neurobiol. 2011; 94:49-63. https://doi.org/10.1016/j.pneurobio.2011.03.006
Chang KH, Vincent F, Shah K. Deregulated Cdk5 triggers aberrant activation of cell cycle kinases and phosphatases inducing neuronal death. J Cell Sci. 2012;125:5124-37. https://doi.org/ 10.1242/jcs.108183
Meyer DA, Torres-Altoro MI, Tan Z, Tozzi A, Di Filippo M, DiNapoli V, et al. Ischemic stroke injury is mediated by aberrant Cdk5. J Neurosci. 2014;34:8259-67. https://doi.org/10.1523/JNEUROSCI.4368-13.2014
Gutiérrez-Vargas JA, Múnera A, Cardona-Gómez GP. CDK5 knockdown prevents hippocampal degeneration and cognitive dysfunction produced by cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:1937-49. https://doi.org/10.1038/jcbfm.2015.150
Khaja AM, Grotta JC. Established treatments for acute ischemic stroke. Lancet. 2007;369:319-30. https://doi.org/10.1016/S0140-6736(07)60154-8
Gutiérrez-Vargas JA, Muñoz-Manco JI, García-Segura LM, Cardona-Gómez GP. GluN2B N-Methyl-D-aspartic acid receptor subunit mediates atorvastatin-induced neuroprotection after focal cerebral ischemia. J Neurosci Res. 2014;92:1529-48. https://doi.org/10.1002/jnr.23426
Piedrahita D, Hernández I, López-Tobón A, Fedorov D, Obara B, Manjunath BS, et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer’s mice. J Neurosci. 2010;30:13966-76. https://doi.org/10.1523/JNEUROSCI.3637-10.2010
Posada-Duque RA, López-Tobón A, Piedrahita D, González-Billault C, Cardona-Gómez GP. p35 and Rac1 underlie the neuroprotection and cognitive improvement induced by CDK5 silencing. J Neurochem.2015;134:354-70. https://doi.org/10.1111/jnc.13127
Posada-Duque RA, Palacio-Castañeda V, Cardona-Gómez GP. CDK5 knockdown in astrocytes provide neuroprotection as a trophic source via Rac1. Mol Cell Neurosci. 2015;68:151-66. https://doi.org/10.1016/j.mcn.2015.07.001
Gutiérrez-Vargas JA, Moreno H, Cardona-Gómez GP. Targeting CDK5 post-stroke provides long-term neuroprotection and rescues synaptic plasticity. J Cereb Blood Flow Metab. 2016;37:2208-23. https://doi.org/0271678X16662476
García JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627-35. https://doi.org/10.1161/01.STR.26.4.627
Chang KH, Multani PS, Sun KH, Vincent F, de Pablo Y, Ghosh S, et al. Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death. Mol Biol Cell. 2011;22:1452-62. https://doi.org/10.1091/mbc.E10-07-0654
Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599-636. https://doi.org/10.1016/0306-4522(91)90001-5
Jin K, Sun Y, Xie L, Peel A, Ou-Mao X, Batteur S, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171-89. https://doi.org/10.1016/S1044-7431(03)00159-3
Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. First edition. Oxford Neuroscience Series. Oxford: Oxford University Press, Inc.; 2007. p. 872.
Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300-6. https://doi.org/10.1161/STROKEAHA.113.001272
Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764-73. https://doi.org/10.1016/j.cub.2013.05.041
Petersen CC. Cortical control of whisker movement. Annu Rev Neurosci. 2014;37:183-203. https://doi.org/10.1146/annurev-neuro-062012-170344
Paus P. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417-24. https://doi.org/10.1038/35077500
Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;16:533-41. https://doi.org/10.1038/nn.4269
Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198-214. https://doi.org/10.1016/j.brainresrev.2006.08.002
Legace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2008;105:18567-71. https://doi.org/10.1073/pnas.0810137105
Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1–DP1 complex. J Neurosci. 2010;30:5219-28. https://doi.org/10.1523/JNEUROSCI.5628-09.2010
Maestre C, Delgado-Esteban M, Gómez-Sánchez JC, Bolaños JP, Almeida A. Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J. 2008;27:2736-45. https://doi.org/10.1038/emboj.2008.195
Artegiani B, Lindemann D, Calegari F. Overexpression of Cdk4 and cyclin D1 triggers greater expansion of neural stem cells in the adult mouse brain. J Exp Med. 2011;208:937-48. https://doi.org/10.1084/jem.20102167
Beukelaers P, Vandenbosch R, Caron N, Nguyen L, Belachew S, Moonen G, et al. Cdk6-dependent regulation of G (1) length controls adult neurogenesis. Stem Cells. 2011;29:713-24. https://doi.org/10.1002/stem.616
Veas-Pérez de Tudela M, Maestre C, Delgado-Esteban M, Bolaños JP, Almeida A. Cdk5-mediated inhibition of APC/C-Cdh1 switches on the cyclin D1-Cdk4-pRb pathway causing aberrant S-phase entry of postmitotic neurons. Sci Rep. 2015;5:18180. https://doi.org/10.1038/srep18180
Niu Y, Li H, Herrup K, Zhang J. Neuronal cell cycle regulation of Cdk5 in Alzheimer’s disease. Brain Disord Ther. 2012;S1:004. https://doi.org/10.4172/2168-975X.S1-004
Zheng YL, Li BS, Rudrabhatla P, Shukla V, Amin ND, Maric D, et al. Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation. Mol Biol Cell. 2010;21:3601-14. https://doi.org/10.1091/mbc.E10-01-0054
Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215-27. https://doi.org/10.1016/S0896-6273(03)00852-3
Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:723. https://doi.org/10.1038/nrn3379
Lu B, Nagappan G, Guan X, Pradeep JN, Wren P. BDNFbased synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:
-16. https://doi.org/10.1038/nrn3505
Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Aktdependent signaling. Neuropharmacology. 2012;63:642-52. https://doi.org/10.1016/j.neuropharm.2012.04.033
Posada-Duque RA, Ramírez O, Härtel S, Inestrosa NC, Bodaleo F, González-Billault C, et al. CDK5 downregulation enhances synaptic plasticity. Cell Mol Life Sci. 2017;74:153. https://doi.org/10.1007/s00018-016-2333-8
Becerra-Calixto A, Cardona-Gómez GP. Neuroprotection induced by transplanted CDK5 knockdown astrocytes in global cerebral ischemic rats. Mol Neurobiol. 2017;54:6681-96. https://doi.org/10.1007/s12035-016-0162-2
Some similar items:
- Jorge Alejandro Henao, Nora Vanegas, Oscar David Cano, Juan Carlos Hiromi, María Teresa Rugeles, The human immunodeficiency virus type 1 and the developing central nervous system. , Biomedica: Vol. 25 No. 1 (2005)
- Lina María De los Reyes, Ángel Enrique Céspedes, Atorvastatin-meloxicam association inhibits neuroinflammation and attenuates the cellular damage in cerebral ischemia by arterial embolism , Biomedica: Vol. 34 No. 3 (2014)
- Angélica María Sabogal, Cesar Augusto Arango, Gloria Patricia Cardona, Ángel Enrique Céspedes, Atorvastatin protects GABAergic and dopaminergic neurons in the nigrostriatal system in an experimental rat model of transient focal cerebral ischemia , Biomedica: Vol. 34 No. 2 (2014)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























