Serosurveillance for vaccine-preventable diseases: A look inside the pertussis experience
Abstract
Introduction: Serological surveillance (serosurveillance) provides the most direct measure of herd immunity of vaccine-preventable diseases. Little is known about the opportunities and challenges of serosurveillance experiences, particularly pertussis.
Objective: To describe the process of serosurveillance for vaccine-preventable diseases with an emphasis on the experience of pertussis in the metropolitan area of Antioquia (Valle de Aburrá) in 2015 and 2016 and analyze the contributions and challenges for its sustainability.
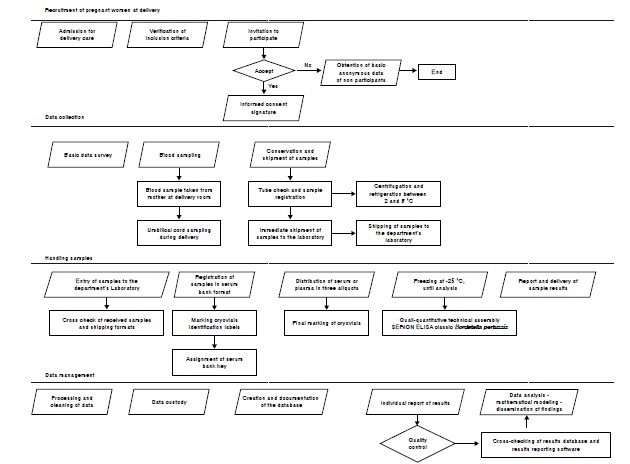
Materials and methods: We described the planning and conduction of serosurveillance of pertussis antibodies of mothers and in the umbilical cord at the time of delivery in eight hospitals based on random sampling and their capacity to advance the serosurveillance periodically. We compared the contributions and the challenges of this experience with other probabilistic and non-probabilistic programs.
Results: We achieved the participation of hospitals and mothers respecting the delivery care process. We established a serum bank following ethical and technical guidelines. This program based on the random selection of hospitals and mothers has enabled the estimation of antibodies prevalence in mothers and in the umbilical cord, which has been possible given the high coverage of hospital care during childbirth at a lower cost and fewer risks than a population-based survey in conflictive areas. The main challenges for the sustainability of this program are the creation of stable jobs and access to funding and legal and methodological long-term frameworks.
Conclusions: Hospital serosurveillance as described is an option to monitor the impact of vaccination on the population. Our experience could be reproduced in other regions under similar conditions if the above-mentioned challenges are solved.
Downloads
References
Domínguez A, Salleras L. Encuestas seroepidemiológicas. Manual de vacunas de pediatría. Madrid: Comité Asesor de Vacunas; 2008. p. 106-17.
Ochoa AR. Técnicas inmunoenzimáticas para ensayos clínicos de vacunas. La Habana: Finlay Ediciones; 2013. p. 65-75.
Cardeñosa-Marín N. Estudios seroepidemiológicos. Revista Española de Salud Pública. 2009;83:607-10.
Paul JR. Serological epidemiology and the function of serum banks. Arch Gesamte Virusforsch. 1965;17:465-71.
Osborne K, Gay N, Hesketh L, Morgan-Capner P, Miller E. Ten years of serological surveillance in England and Wales: Methods, results, implications and action. Int J Epidemiol. 2000;29:362-8.
Magos C, Sánchez F, Gutiérrez G, Tapia R. Banco nacional de sueros. Salud Pública de México. 1992;34:136-47.
Evans A. Surveillance and seroepidemiology. In: Alfred E, editor. Viral Infections of Humans: Epidemiology and control. New York and London: Plenum Medical Book Company; 1983. p. 43-64.
Metcalf CJ, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388:728-30. https://doi.org/10.1016/S0140-6736(16)30164-7
Cutts FT, Hanson M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop Med Int Health. 2016;21:1086-98. https://doi.org/10.1111/tmi.12737
Wilson SE, Deeks SL, Hatchette TF, Crowcroft NS. The role of seroepidemiology in the comprehensive surveillance of vaccine-preventable diseases. CMAJ. 2012;184:E70-6. https://doi.org/10.1503/cmaj.110506
Ministerio de Salud y Protección Social, Instituto Nacional de Salud. Lineamiento estratégico para la introducción de la vacuna DPaT (Difteria - tos ferina acelular -tétanos) en el esquema del Programa Ampliado de Inmunizaciones - PAI para mujeres gestantes de las cohortes 2013 y 2014, Colombia, 2013. Bogotá: Ministerio de Salud y Protección Social; 2013. p. 29.
Hincapié-Palacio D, Hoyos MC, Ochoa J, Montoya N, García D, Osorio E. Effect of maternal immunization against pertussis in Medellín and the metropolitan area, Colombia, 2016-2017. Vaccine. 2018;36:3984-91. https://doi.org/10.1016/j.vaccine.2018.05.020
Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, et al. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32:1257-60. https://doi.org/10.1097/INF.0b013e3182a09b6a
Plans P, Jansa J, Doshi N, Harrison TG, Plasencia A. Prevalence of pertussis antibodies in umbilical cord blood samples in Catalonia, Spain. Pediatr Infect Dis J. 2008;27:1023-5. https://doi.org/10.1097/INF.0b013e318179264b
Nooitgedagt JE, de Greeff SC, Elvers BH, de Melker HE, Notermans DW, van Huisseling H, et al. Seroprevalence of Bordetella pertussis infection during pregnancy measured by IgG antibodies against pertussis toxin. Clin Infect Dis. 2009;49:1086-9. https://doi.org/10.1086/605575
Riffelmann M, Thiel K, Schmetz J, Wirsing von Koenig CH. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. J Clin Microbiol. 2010;48:4459-63. https://doi.org/10.1128/JCM.01371-10
Magos-López C, Sánchez-Villarreal F, Gutiérrez G, Tapia-Conyer R. Banco Nacional de Sueros. Salud Pública México. 1992;31:136-47.
Hernández-Ávila M. Resultados de serología de la Encuesta Nacional de Salud 2000. Salud Pública de México. 2007;49:321-3.
van der Klis FR, Mollema L, Berbers GA, de Melker HE, Coutinho RA. Second national serum bank for population-based seroprevalence studies in the Netherlands. Neth J Med. 2009;67:301-8.
De Melker HE, Conyn-van Spaendonck MA. Immunosurveillance and the evaluation of national immunization programmes: A population-based approach. Epidemiol Infect. 1998;121:637-43.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: Plan and operations, 1999-2010. Vital Health Stat 1. 2013;56:1-37.
Introcaso CE, Dunne EF, Hariri S, Panicker G, Unger ER, Markowitz LE. Prevaccine era human papillomavirus types 6, 11, 16 and 18 seropositivity in the U.S.A., National Health and Nutrition Examination Surveys, 2003-2006. Sex Transm Infect. 2014;90:505-8. https://doi.org/10.1136/sextrans-2013-051490
Liu B, Taioli E. Associations between human papillomavirus and history of cancer among U.S. adults in the National Health and Nutrition Examination Survey (2003-2010). Br J Cancer. 2014;111:1448-53. https://doi.org/10.1038/bjc.2014.414
Kruszon-Morán D, Klevens RM, McQuillan GM. Change in hepatitis A seroprevalence among U.S. children and adolescents: Results from the National Health and Nutrition Examination Survey 2003-2006 and 2007-2010. Vaccines (Basel). 2013;1:105-19. https://doi.org/10.3390/vaccines1020105
Dhankhar P, Nwankwo C, Pillsbury M, Lauschke A, Goveia MG, Acosta CJ, et al. Public health impact and cost-effectiveness of hepatitis a vaccination in the United States: A disease transmission dynamic modeling approach. Value Health. 2015;18:358-67. https://doi.org/10.1016/j.jval.2015.02.004
Hincapié-Palacio D, Lenis-Ballesteros V, Ospina MO, Toro OL, Díaz FJ. Seroprevalence of rubella in Colombia: A birth-year cohort analysis. Rev Saúde Pública. 2013;47:1080-91. https://doi.org/10.1590/S0034-8910.2013047004749
Hincapié-Palacio D, Ospina-Giraldo J, Lenis-Ballesteros V, Ospina-Ospina MC, Arroyave-Cadavid M, Hoyos-Muñoz N, et al. Inmunidad colectiva contra la rubéola según una encuesta poblacional en Medellín, Colombia. Rev Panam Salud Pública. 2012;32:101–8. https://doi.org/10.1590/s1020-49892012000800003
Santacruz-Sanmartín E, Hincapié-Palacio D, Ospina MC, Pérez-Toro O, Bernal-Restrepo LM, Buitrago-Giraldo S, et al. Seroprevalence of mumps in an epidemic period in Medellín, Colombia. Vaccine. 2015;33:5606-12. https://doi.org/10.1016/j.vaccine.2015.08.088
Cadavid D, Hincapié-Palacio D, Ospina M, Bernal L, Buitrago S, Pérez O, et al. Status for hepatitis B virus infection and socioeconomic variables: A multiple correspondence analysis. J Viral Hepat. 2014;21:36-7. https://doi.org/10.1111/jvh.12333_27
Cadavid-Betancur DA, Ospina MC, Hincapié-Palacio D, Bernal-Restrepo LM, Buitrago-Giraldo S, Pérez-Toro O, et al. Seroprevalence of hepatitis B and factors potentially associated in a population-based study in Medellín, Colombia. Vaccine. 2017;35:4905-12. https://doi.org/10.1016/j.vaccine.2017.07.084
Hincapié-Palacio D, Ospina-Giraldo J, Gómez-Árias RD, Uyi-Afuwape A, Chowell-Puente G. Simulating measles and rubella elimination levels according to social stratification and interaction. Rev Salud Pública (Bogotá). 2010;12:103-15.
Gidding H. Australia’s national serosurveillance program. N S W Public Health Bull. 2003;14:90-3. https://doi.org/10.1071/NB03027
Jardine A, Deeks SL, Patel MS, Menzies RI, Gilbert GL, McIntyre PB. An evaluation of the Australian National Serosurveillance Program. Commun Dis Intell Q Rep. 2010;34:29-36.
Osborne K, Weinberg J, Miller E. The European Sero-Epidemiology Network. Euro Surveill. 1997;2:29-31.
Andrews N, Tischer A, Siedler A, Pebody RG, Barbara C, Cotter S, et al. Towards elimination: Measles susceptibility in Australia and 17 European countries. Bull World Health Organ. 2008;86:197-204. https://doi.org/10.2471/BLT.07.041129
Sutton EF, Cain LE, Vallo PM, Redman LM. Strategies for successful recruitment of pregnant patients into clinical trials. Obstet Gynecol. 2017;129:554-9. https://doi.org/10.1097/aog.0000000000001900
Ospina J, Hincapié-Palacio D. The critical proportion of immune individuals needed to control hepatitis B. Proceedings of SPIE. 2016;9863:1-10. https://doi.org/10.1117/12.2222129
Departamento Administrativo Nacional de Estadística. Nacimientos Bogotá: DANE. Accessed on: October 20, 2017. Available from: http://www.dane.gov.co/index.php/estadisticas-por-tema/salud/nacimientos-y-defunciones/nacimientos/nacimientos-2015
de Voer RM, van der Klis FR, Nooitgedagt JE, Versteegh FG, van Huisseling JC, van Rooijen DM, et al. Seroprevalence and placental transportation of maternal antibodies specific for Neisseria meningitidis serogroup C, Haemophilus influenzae type B, diphtheria, tetanus, and pertussis. Clin Infect Dis. 2009;49:58-64. https://doi.org/10.1086/599347
Laurie KL, Huston P, Riley S, Katz JM, Willison DJ, Tam JS, et al. Influenza serological studies to inform public health action: Best practices to optimise timing, quality and reporting. Influenza Other Respir Viruses. 2013;7:211-24. https://doi.org//10.1111/j.1750-2659.2012.0370a.x
Tafuri S, Gallone MS, Gallone MF, Cappelli MG, Chironna M, Germinario C. Evaluation of a vaccination strategy by serosurveillance data: The case of varicella. Hum Vaccin Immunother. 2015;11:897-900. https://doi.org/10.1080/21645515.2015.1009818
Barkoff AM, Grondahl-Yli-Hannuksela K, He Q. Seroprevalence studies of pertussis: What have we learned from different immunized populations. Pathog Dis. 2015;73. https://doi.org/10.1093/femspd/ftv050
Gonik B, Puder KS, Gonik N, Kruger M. Seroprevalence of Bordetella pertussis antibodies in mothers and their newborn infants. Infect Dis Obstet Gynecol. 2005;13:59-61. https://doi.org/10.1080/10647440500068289
Some similar items:
- Ana María Perilla, Camilo González, Sandra Liliana Valderrama, Natasha Vanegas, Bibiana Chavarro, Luis Carlos Triana, José Roberto Támara, Carlos Arturo Álvarez, Necrotizing pneumonia by community-acquired, methicillin-resistant Staphylococcus aureus in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Yolanda Lucía López, Claudia González, Berta Natalia Gallego, Ana Lida Moreno, Stewardship of public health surveillance in the health system in Colombia: a cases study , Biomedica: Vol. 29 No. 4 (2009)
- Oscar Fernando Herrán, María F. Ardila, Categories of alcohol consumers and the criteria for classification , Biomedica: Vol. 29 No. 4 (2009)
- Ingrid Yamile Pulido, José Ramón Mantilla, Emilia María Valenzuela, María Teresa Reguero, Elsa Beatriz González, Distribution of extended spectrum β-lactamases-codifying genes in Klebsiella pneumoniae isolates from hospitals of Bogota, D.C., Colombia , Biomedica: Vol. 31 No. 1 (2011)
- Greizy López, Nancy Yaneth Gelvez, Martalucía Tamayo, Mutational frequencies in usherin (USH2A gene) in 26 Colombian individuals with Usher syndrome type II , Biomedica: Vol. 31 No. 1 (2011)
- Jhon Carlos Castaño, Fidel Ángel Núñez, María Mercedes González, Germán Téllez, María Isabel Giraldo, First case report of Mammomonogamus (Syngamus) laryngeus human infection in Colombia , Biomedica: Vol. 26 No. 3 (2006)
- Andrés F. Londoño, Silvana Levis, Juan D. Rodas, Hantavirus as important emerging agents in South America , Biomedica: Vol. 31 No. 3 (2011)
- Jefferson Antonio Buendía, Attitudes, knowledge and beliefs of patient about anti-hypertensive drugs , Biomedica: Vol. 32 No. 4 (2012)
- Mauricio Beltrán, Maritza Berrío-Pérez, María Isabel Bermúdez, Gloria Rey-Benito, Bernardo Camacho, Patricia Forero, Gloria Cristina Molina, Orlando Fals, Isabel Pisciotti, Yulieth Oliveros, Armando Cortés, Fernando De La Hoz, Absence of occult hepatitis B in Colombian blood donors , Biomedica: Vol. 31 No. 4 (2011)
- Pablo Chaparro, Edison Soto, Julio Padilla, Daniel Vargas, Estimation of the underreporting of malaria measurement in ten municipalities of the Pacific coast of Nariño during 2009 , Biomedica: Vol. 32 (2012): Suplemento 1, Malaria
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























