Quantitative analysis of the expression of fluconazole-resistant genes in strains of Candida albicans isolated from elderly people at their admission in an intensive care unit in Manizales, Colombia
Abstract
Introduction: Opportunistic infections associated with Candida albicans have had a great impact on public health due to the mortality they generate in certain population groups. Although pharmacological treatments are available, the resistance developed by the pathogen has become increasingly evident. For this reason, determining the mechanisms of resistance associated with the strains found in different hospital areas is important since it would help improving treatment plans.
Objective: To analyze the expression of ERG11, CDR1, and MDR1 genes in strains of C. albicans isolated from elderly patients at admittance in the intensive care unit of Hospital Santa Sofía in Manizales, Colombia.
Materials and methods: A total of 29 samples (21 resistant and 8 sensitive) were selected and distributed in two working groups: with and without exposure to fluconazole. The extracted RNA was quantified by real-time reverse transcription polymerase chain reaction (RT-qPCR).
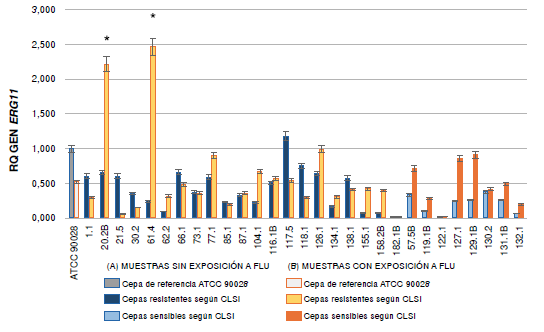
Results: Significant differences were found in the expression of the MDR1 gene in the group of resistant C. albicans strains. Two of the resistant strains (104 and 62-2) exposed to the antifungal showed very high values in the expression of this gene. The expression of ERG11 and CDR1 was not significant among the groups studied.
Conclusion: The increased overexpression of the MDR1 gene indicates that it may be responsible for the resistance. However, some resistant strains did not overexpress any of the genes analyzed, which indicates that there may be other genes involved in the resistance of the strains under study.
Downloads
References
Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753-61. https://doi.org/10.1016/j.micinf.2009.04.018
Andes D. Clinical utility of antifungal pharmacokinetics and pharmacodynamics. Curr Opin Infect Dis. 2004;17:533-40.
Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503-35. https://doi.org/10.1086/596757
Cortés JA, Reyes P, Gómez C, Buitrago G, Leal AL. Fungal bloodstream infections in tertiary care hospitals in Colombia. Rev Iberoam Micol. 2011;28:74-8. https://doi.org/10.1016/j.riam.2010.12.002
De Bedout C, Ayabaca J, Vega R, Méndez M, Santiago ÁR. Evaluación de la susceptibilidad de especies de Candida al fluconazol por el método de difusión de disco. Biomédica. 2003;23:31-7. https://doi.org/10.7705/biomedica.v23i1.1195
Gutiérrez C, De Bedout C, Tobón AM, Cano LE, Arango M, Tabares AM, et al. Sensibilidad a fluconazol y voriconazol de aislamientos de Candida spp., obtenidos de mucosa oral de pacientes con sida. Infectio. 2008;11:183-9.
Duque C, Gómez B, Uribe O, Alarcón J, Soto F, Urán L, et al. Caracterización de la candidiasis vulvovaginal en mujeres de la ciudad de Medellín, Colombia. Nova. 2009;7:157-60.
Maldonado NA, Cano LE, De Bedout C, Arbeláez CA, Roncancio G, Tabares AM, et al. Association of clinical and demographic factors in invasive candidiasis caused by fluconazoleresistant Candida species: A study in 15 hospitals, Medellín, Colombia 2010-2011. Diagn Microbiol Infect Dis. 2014;79:280-6. https://doi.org/10.1016/j.diagmicrobio.2014.02.003
Hernández JS. Estudio básico-clínico de la colonización de especies de candida en adultos mayores al ingreso de cuidados intensivos (tesis). Manizales: Universidad de Caldas; 2015.
Kanafani ZA, Perfect JR. Resistance to antifungal agents: Mechanisms and clinical impact. Clin Infect Dis. 2008;46:120-8. https://doi.org/10.1086/524071
Pemán J, Cantón E, Espinel-Ingroff A. Antifungal drug resistance mechanisms. Expert Rev Anti Infect Ther. 2009;7:453-60. https://doi.org/10.1586/eri.09.18
Rocha MF, Bandeira SP, De Alencar LP, Melo LM, Sales JA, Paiva M de AN, et al. Azole resistance in Candida albicans from animals: Highlights on efflux pump activity and gene overexpression. Mycoses. 2017;60:462-8. https://doi.org/10.1111/myc.12611
Mandal A, Kumar A, Singh A, Lynn AM, Kapoor K, Prasad R. A key structural domain of the Candida albicans Mdr1 protein. Biochem J. 2012;445:313-22. https://doi.org/10.1042/BJ20120190
White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate, with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482-7. https://doi.org/10.1128/AAC.41.7.1482
Joseph-Horne T, Hollomon DW. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149:141-9. https://doi.org/10.1111/j.1574-6968.1997.tb10321.x
Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002;359:1135-44. https://doi.org/10.1016/S01406736(02)08162-X
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-22. https://doi.org/10.1373/clinchem.2008.112797
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeast. Third edition. Wayne: CLSI; 2008.
Chau AS, Mendrick CA, Sabatelli FJ, Mcnicholas PM, Loebenberg D. Application of realtime quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob Agents Chemother. 2004;48:2124-31. https://doi.org/10.1128/AAC.48.6.2124-2131.2004
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402-8. https://doi.org/10.1006/meth.2001.1262
Zuluaga A, de Bedout C, Agudelo CA, Hurtado H, Arango M, Restrepo Á, et al. Sensibilidad a fluconazol y voriconazol de especies de Candida aisladas de pacientes provenientes de unidades de cuidados intensivos en Medellín, Colombia (2001-2007). Rev Iberoam Micol. 2010;27:125-9. https://doi.org/10.1016/j.riam.2010.04.001
White TC, Holleman S, Dy F, Stevens DA, Mirels LF. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704-13. https://doi.org/10.1128/AAC.46.6.1704-1713.2002
Perea S, López-Ribot JL, Kirkpatrick WR, Mcatee RK, Santillán RA, Martínez M, et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001;45:2676-84. https://doi.org/10.1128/AAC.45.10.2676–2684.2001
Mane A, Vidhate P, Kusro C, Waman V, Saxena V, Kulkarni-Kale U, et al. Molecular mechanisms associated with fluconazole resistance in clinical Candida albicans isolates from India. Mycoses. 2016;59:93-100. https://doi.org/10.1111/myc.12439
Salari S, Khosravi AR, Mousavi SAA, Nikbakht-Brojeni GH. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J Mycol Med. 2016;26:35-41. https://doi.org/10.1016/j.mycmed.2015.10.007
Tavakoli M, Zaini F, Kordbacheh M, Safara M, Raoofian R, Heidari M. Upregulation of the ERG11 gene in Candida krusei by azoles. Daru. 2010;18:276-80.
Hiller D, Sanglard D, Morschhauser J. Overexpression of the MDR1 gene is sufficient to confer increased resistance to toxic compounds in Candida albicans. Antimicrob Agents Chemother. 2006;50:1365-71. https://doi.org/10.1128/AAC.50.4.1365-1371.2006
Correa RA. Evaluación de mutaciones del gen ERG11 como causa de resistencia al fluconazol en aislamientos clinicos de pacientes colonizados por C. albicans obtenidas de adultos mayores en la unidad de cuidado intensivo del Hospital Santa Sofía de Manizales - Colombia (tesis). Manizales: Universidad de Caldas; 2016.
Franz R, Kelly SL, Lamb DC, Kelly DE, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065-72. https://doi.org/10.1128/AAC.42.12.3065
Franz R, Ruhnke M, Morschhäuser J. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses. 1999;42:453-8. https://doi.org/10.1046/j.1439-0507.1999.00498.x
López-Ribot JL, McAtee RK, Lee LN, Kirkpatrick WR, White TC, Sanglard D, et al. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932-7. https://doi.org/10.1128/AAC.42.11.2932
Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378-86. https://doi.org/10.1128/AAC.39.11.2378
Khosravi Rad K, Falahati M, Roudbary M, Farahyar S, Nami S. Overexpression of MDR-1 and CDR-2 genes in fluconazole resistance of Candida albicans isolated from patients with vulvovaginal candidiasis. Curr Med Mycol. 2016;2:24-9. https://doi.org/10.18869/acadpub.cmm.2.4.24
Wirsching S, Michel S, Köhler G, Morschhäuser J. Activation of the multiple drug resistance gene MDR1 in fluconazole-resistant, clinical Candida albicans strains is caused by mutations in a trans-regulatory factor. J Bacteriol. 2000;182:400-4. https://doi.org/10.1128/JB.182.2.400-404.2000
Wirsching S, Michel SM. Targeted gene disruption in Candida albicans wild-type strains: The role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol. 2000;36:856-65. https://doi.org/10.1046/j.1365-2958.2000.01899.x
Wirsching S, Moran GP, Sullivan DJ, Coleman DC. MDR1-mediated drug resistance in Candida dubliniensis. Antimicrob Agents Chemother. 2001;45:3416-21. https://doi.org/10.1128/AAC.45.12.3416-3421.2001
Karababa M, Coste AT, Rognon B, Bille J, Sanglard D. Comparison of gene expression profiles of Candida albicans azole-resistant clinical isolates and laboratory strains exposed to drugs inducing multidrug transporters. Antimicrob Agents Chemother. 2004;48:3064-79. https://doi.org/10.1128/AAC.48.8.3064-3079.2004
Kusch H, Biswas K, Schwanfelder S, Engelmann S, Rogers PD, Hecker M, et al. A proteomic approach to understanding the development of multidrug-resistant Candida albicans strains. Mol Genet Genomics. 2004;271:554-65. https://doi.org/10.1007/s00438-004-0984-x
Rogers PD, Barker KS. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Society. 2003;47:1220-7. https://doi.org/10.1128/AAC.47.4.1220-1227.2003
Park S, Perlin DS. Establishing surrogate markers for fluconazole resistance in Candida albicans. Microb drug Resist. 2005;11:232-8. https://doi.org/10.1089/mdr.2005.11.232
Watamoto T, Samaranayake LP, Egusa H, Yatani H, Seneviratne CJ. Transcriptional regulation of drug-resistance genes in Candida albicans biofilms in response to antifungals. J Med Microbiol. 2011;60:1241-7. https://doi.org/10.1099/jmm.0.030692-0
Morschhäuser J, Barker KS, Liu TT, BlaB-Warmuth J, Homayouni R, Rogers PD. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007;3:1603-16. https://doi.org/10.1099/jmm.0.030692-0
Eddouzi J, Parker JE, Vale-Silva LA, Coste A, Ischer F, Kelly S, et al. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob Agents Chemother. 2013;57:3182-93. https://doi.org/10.1128/AAC.00555-13
Marchaim D, Lemanek L, Sobel JD, Kaye KS. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol. 2012;120:1407-14. https://doi.org/10.1097/AOG.0b013e31827307b2
Some similar items:
- Ana Elisa Rojas, Leidy Yurany Cárdenas, María Camila García, Jorge Enrique Pérez, Expression of ERG11, ERG3, MDR1 and CDR1 genes in Candida tropicalis , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Husein Husein-El Ahmed, Guillermo Arturo Cañadas-De la Fuente, Rafael Fernández-Castillo, Emilio González-Jiménez, Jesús Cantero-Hinojosa, Marita Lardón-Fernández, Generalized cutaneous candidiasis in newborn at term , Biomedica: Vol. 32 No. 2 (2012)
- Catalina de Bedout, Julio Ayabaca, Ricardo Vega, Matilde Méndez, Axel R. Santiago, María Lucrecia Pabón, Angela Tabares, Myrtha Arango, Angela Restrepo, Vance Newell, Evaluation of Candida species' susceptibility to fluconazole with the disk diffusion method. , Biomedica: Vol. 23 No. 1 (2003)
- Liliana Torcoroma García, Liany Johanna Luna, Tania Katherine Velasco, Beatriz Elena Guerra, new multiplex PCR for species-specific diagnosis of human candidiasis , Biomedica: Vol. 37 No. 2 (2017)
- Isaura Torres, Juan E. Gallo , Oscar Mauricio Gómez , Álvaro Rúa-Giraldo , Juan G. McEwen , Ana María García , Gene expression profiles of ERG11, MDR1 and AFR1 in Cryptococcus neoformans var.grubbi from HIV patients , Biomedica: Vol. 42 No. 4 (2022)
- Javier Araiza , Valentín Sánchez-Pedraza, Ana Karen Carrillo , Denise Fernández-Samar, Jazmín Tejeda, Alexandro Bonifaz, Mixed oral candidiasis in type 2 diabetic patients: Identification and spectrum of sensitivity , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Kevin Ehemann, Andrés Contreras, Adriana Marcela Celis-Ramírez, In vitro sensitivity of Malassezia furfur isolates from HIV-positive and negative patients to antifungal agents , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Soraya Morales-López, Keiner Ustate, Zulay Pedrozo, Yulibeth Torres, Biochemical typing and evaluation of pathogenicity in vulvovaginal isolates of Candida albicans complex , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- James Alexander Castillo , Natalia Afanasjeva, Method validation for the quantification of fluconazole and its organic impurities in raw material using high-performance liquid chromatography , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























