Salivary markers of oxidative stress and periodontal pathogens in patients with periodontitis from Santander, Colombia
Abstract
Introduction: Periodontitis affects more than 20% of the Latin American population.
Oxidative markers are associated with greater progression of periodontitis; therefore, its role in pathogenesis should be studied.
Objective: To determine the prevalence of the main oral bacteria and viruses associated with periodontitis and estimate the total antioxidant capacity and lipid peroxidation in saliva from patients with periodontitis.
Materials and methods: We conducted systemically a cross-sectional study in 101 healthy subjects, 87 of whom had been diagnosed with periodontitis (P), according to the criteria of the Centers of Disease Control and Prevention and the American Academy of Periodontology, and 14 without periodontal pockets as controls (C). In subgingival samples, major viruses and dental pathogenic bacteria were identified using PCR techniques. The levels of total antioxidant capacity and malon-di-aldehyde (MDA) were determined by spectrophotometry in samples of unstimulated saliva.
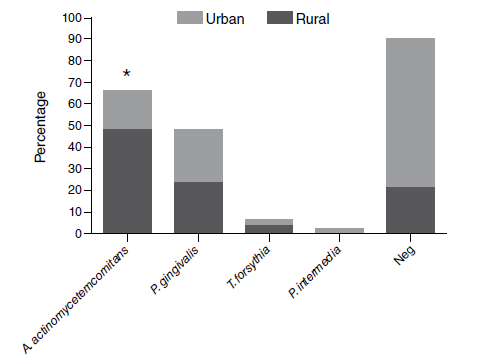
Results: The mean of periodontal depth pocket and clinical attachment loss in patients with periodontitis was 5.6 ± 1.7 and 6.1 ± 3.1 mm, respectively. The most prevalent microorganisms were Aggregatibacter actinomycetemcomitans (32.5%) and Porphyromonas gingivalis (18.6%). The patients from rural areas showed a higher percentage of A. actinomycetemcomitans (urban: 17.9% vs. rural: 48.9%, p=0.0018). In patients with periodontitis, the frequency of EBV, HSV1 & 2, and HCMV genes was 2.3%. Periodontitis patients had higher levels of MDA (P: 2.1 ± 1.5; C: 0.46 ± 0.3 μmol/g protein; p=0.0001) and total antioxidant capacity (P: 0.32 ± 0.2; C: 0.15 ± 0.1 mM; p< 0.0036). Oxidative markers showed no modifications due to the presence of periodontopathic bacteria.
Conclusions: Aggregatibacter actinomycetemcomitans was the most prevalent bacteria; its presence did not modify the levels of oxidative markers in the saliva of patients with periodontitis.
Downloads
References
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809-20. https://doi.org/10.1016/S0140-6736(05)67728-8
Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44 (Suppl.18):S94-S105. https://doi.org/10.1111/jcpe.12677
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J Dent Res. 2014;93:1045-53. https://doi.org/10.1177/0022034514552491
Ministerio de Salud y Protección Social. IV Estudio Nacional de Salud Bucal ENSAB-IV 2013-2014. Bogotá: Minsalud; 2014.
Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl.18):S39-S51. https://doi.org/10.1111/jcpe.12685
Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. 2017;8:910. https://doi.org/10.3389/fphys.2017.00910
Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: Understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. http://doi.org/10.3389/fphys.2017.00693
Lafaurie GI, Contreras A, Barón A, Botero J, Mayorga-Fayad I, Jaramillo A, et al. Demographic, clinical, and microbial aspects of chronic and aggressive periodontitis in Colombia: A multicenter study. J Periodontol. 2007;78:629-39. https://doi.org/10.1902/jop.2007.060187
Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160-232. https://doi.org/10.1111/j.1600-0757.2006.00178.x
Banasova L, Kamodyova N, Jansakova K, Tothova L, Stanko P, Turna J, et al. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin Oral Investig. 2015;19:201-7. https://doi.org/10.1007/s00784-014-1236-z
Slots J. Herpesviruses, the missing link between gingivitis and periodontitis? J Int Acad Periodontol. 2004;6:113-9.
Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7Suppl.):1387-99. https://doi.org/10.1902/jop.2007.060264
Ghizoni JS, Taveira LA, Garlet GP, Ghizoni MF, Pereira JR, Dionisio TJ, et al. Increased levels of Porphyromonas gingivalis are associated with ischemic and hemorrhagic cerebrovascular disease in humans: An in vivo study. J Appl Oral Sci. 2012;20:104-12. https://doi.org/10.1590/s1678-77572012000100019
Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266-73. https://doi.org/10.1111/j.1399-302X.1996.tb00180.x
Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36-47. https://doi.org/10.1111/j.1600-0757.2010.00350.x
Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66-77. https://doi.org/10.1111/j.1600-0757.1994.tb00019.x
Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, et al. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J Clin Periodontol. 2008;35:106-13. https://doi.org/10.1111/j.1600-051X.2007.01170.x
Sanz M, van Winkelhoff AJ, Herrera D, Dellemijn-Kippuw N, Simon R, Winkel E. Differences in the composition of the subgingival microbiota of two periodontitis populations of different geographical origin. A comparison between Spain and The Netherlands. Eur J Oral Sci. 2000;108:383-92. https://doi.org/10.1034/j.1600-0722.2000.108005383.x
Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648-57. https://doi.org/10.1034/j.1600-051x.2000.027009648.x
Mayorga-Fayad I, Lafaurie GI, Contreras A, Castillo DM, Barón A, Aya Mdel R. Subgingival microbiota in chronic and aggressive periodontitis in Bogotá, Colombia: An epidemiological approach. Biomédica. 2007;27:21-33. https://doi.org/10.7705/biomedica.v27i1.230
Ardila-Medina CM, Arbeláez-Montoya MI, Guzmán-Zuluaga IC. Perfil microbiológico subgingival de pacientes con periodontitis crónica en una población de Colombia. Avances en Periodoncia. 2012;24:47-53.
Kakuta E, Nomura Y, Morozumi T, Nakagawa T, Nakamura T, Noguchi K, et al. Assessing the progression of chronic periodontitis using subgingival pathogen levels: A 24-month prospective multicenter cohort study. BMC Oral Health. 2017;17:46. https://doi.org/10.1186/s12903-017-0337-x
Zambon JJ, Christersson LA, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. J Periodontol. 1983;54:707-11. https://doi.org/10.1902/jop.1983.54.12.707
Jardim Junior EG, Bosco JM, Lopes AM, Landucci LF, Jardim EC, Carneiro SR. Occurrence of Actinobacillus actinomycetemcomitans in patients with chronic periodontitis, aggressive periodontitis, healthy subjects and children with gingivitis in two cities of the state of Sao Paulo, Brazil. J Appl Oral Sci. 2006;14:153-6.
https://doi.org/10.1590/s1678-77572006000300001
Okada M, Hayashi F, Nagasaka N. Detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in dental plaque samples from children 2 to 12 years of age. J Clin Periodontol. 2000;27:763-8. https://doi.org/10.1034/j.1600-051x.2000.027010763.x
López NJ, Mellado JC, Leighton GX. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia in juvenile periodontitis. J Clin Periodontol. 1996;23:101-5. https://doi.org/10.1111/j.1600-051X.1996.tb00541.x
Mombelli A, Casagni F, Madianos PN. Can presence or absence of periodontal pathogens distinguish between subjects with chronic and aggressive periodontitis? A systematic review. J Clin Periodontol. 2002;29(Suppl.3):10-21. https://doi.org/10.1034/j.1600-051X.29.s3.1.x
Botero JE, Parra B, Jaramillo A, Contreras A. Subgingival human cytomegalovirus correlates with increased clinical periodontal parameters and bacterial coinfection in periodontitis. J Periodontol. 2007;78:2303-10. https://doi.org/10.1902/jop.2007.070252
Riggio MP, Macfarlane TW, Mackenzie D, Lennon A, Smith AJ, Kinane D. Comparison of polymerase chain reaction and culture methods for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in subgingival plaque samples. J Periodontal Res. 1996;31:496-501. https://doi.org/10.1111/j.1600-0765.1996.tb01415.x
Jaramillo A, Arce R, Contreras A, Herrera JA. Efecto del tratamiento periodontal sobre la microbiota subgingival en pacientes con preeclampsia. Biomédica. 2012;32:233-8. https://doi.org/10.7705/biomedica.v32i2.661
Michalowicz BS, Ronderos M, Camara-Silva R, Contreras A, Slots J. Human herpesviruses and Porphyromonas gingivalis are associated with juvenile periodontitis. J Periodontol. 2000;71:981-8. https://doi.org/10.1902/jop.2000.71.6.981
Cappuyns I, Gugerli P, Mombelli A. Viruses in periodontal disease - a review. Oral Dis. 2005;11:219-29. https://doi.org/10.1111/j.1601-0825.2005.01123.x
Aljehani YA. Risk factors of periodontal disease: Review of the literature. Int J Dent. 2014;2014:9.https://doi.org/10.1155/2014/182513
Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017;11:72-80.
Nunes LA, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem Med (Zagreb). 2015;25:177-92. https://doi.org/10.11613/BM.2015.018
Onder C, Kurgan S, Altingoz SM, Bagis N, Uyanik M, Serdar MA, et al. Impact of nonsurgical periodontal therapy on saliva and serum levels of markers of oxidative stress. Clin Oral Investig. 2017;21:1961-9. https://doi.org/10.1007/s00784-016-1984-z
Sawamoto Y, Sugano N, Tanaka H, Ito K. Detection of periodontopathic bacteria and an oxidative stress marker in saliva from periodontitis patients. Oral Microbiol Immunol. 2005;20:216-20. https://doi.org/10.1111/j.1399-302X.2005.00215.x
Ahmadi-Motamayel F, Goodarzi MT, Jamshidi Z, Kebriaei R. Evaluation of salivary and serum antioxidant and oxidative stress statuses in patients with chronic periodontitis: A casecontrol study. Front Physiol. 2017;8:189. https://doi.org/10.3389/fphys.2017.00189
Khalili J, Biloklytska HF. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008;14:754-60. https://doi.org/10.1111/j.1601-0825.2008.01464.x
Nguyen TT, Ngo LQ, Promsudthi A, Surarit R. Salivary lipid peroxidation in patients with generalized chronic periodontitis and acute coronary syndrome. J Periodontol. 2016;87:134-41. https://doi.org/10.1902/jop.2015.150353
Almerich-Silla JM, Montiel-Company JM, Pastor S, Serrano F, Puig-Silla M, Dasi F. Oxidative stress parameters in saliva and its association with periodontal disease and types of bacteria. Dis Markers. 2015;2015:7. https://doi.org/10.1155/2015/653537
Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515-21. https://doi.org/10.1111/j.1600-051X.2004.00509.x
Su H, Gornitsky M, Velly AM, Yu H, Benarroch M, Schipper HM. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic Biol Med. 2009;46:914-21. https://doi.org/10.1016/j.freeradbiomed.2009.01.008
Kamodyova N, Tothova L, Celec P. Salivary markers of oxidative stress and antioxidant status: Influence of external factors. Dis Markers. 2013;34:313-21. https://doi.org/10.3233/DMA-130975
Some similar items:
- Martine Bonnaure-Mallet, Paula Juliana Pérez-Chaparro, Patrice Gracieux, Vincent Meuric, Zohreh Tamanai-Shacoori, Jaime Eduardo Castellanos, Distribution of Porphyromonas gingivalis fimA genotypes in isolates from subgingival plaque and blood sample during bacteremia , Biomedica: Vol. 29 No. 2 (2009)
- Isabel Mayorga-Fayad, Gloria I. Lafaurie, Adolfo Contreras, Diana M. Castillo, Alexandra Barón, María del Rosario Aya, Subgingival microbiota in chronic and aggressive periodontitis in Bogotá, Colombia: an epidemiological approach , Biomedica: Vol. 27 No. 1 (2007)
- Andrea Arévalo, Julio César Carranza, Felipe Guhl, Gustavo Adolfo Vallejo, Electrophoretic patterns of salivary hemeproteins (nitrophorines) of Rhodnius colombiensis and R. prolixus (Hemiptera, Reduviidae, Triatominae) , Biomedica: Vol. 27 No. 1esp (2007): Enfermedad de Chagas
- Elsa Nieves, Neudo Buelvas, Maritza Rondón, Néstor González, The salivary glands of two sand fly vectors of Leishmania: Lutzomyia migonei (França) and Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) , Biomedica: Vol. 30 No. 3 (2010)
- Jessika Consuegra, Sonia Jakeline Gutiérrez, Adriana Jaramillo, Ignacio Sanz, Gilberto Olave, Jorge Enrique Soto, Carlos Valencia, Adolfo Contreras, Enteric Gram negative rods and unfermented of glucose bacteria in patients with peri-implant disease , Biomedica: Vol. 31 No. 1 (2011)
- Edisson Rodríguez, Aura María Gil-Villa, Daniel Camilo Aguirre-Acevedo, Walter Cardona-Maya, Ángela P. Cadavid, Evaluation of atypical semen parameters in individuals whose couples had a history of early recurrent embryo death: in search for a reference value , Biomedica: Vol. 31 No. 1 (2011)
- Sandra Paola Ochoa, Carlos Andrés Ospina, Kelly Johana Colorado, Yenny Paola Montoya, Andrés Fernando Saldarriaga, Marisol Miranda, Natalia Muñoz, María Eugenia Gómez, Fanny Lucía Yepes, Javier Enrique Botero, Periodontal condition and tooth loss in diabetic patients , Biomedica: Vol. 32 No. 1 (2012)
- Andrés Páez, Constanza Nuñez, Clemencia García, Jorge Boshell, Molecular epidemiology of rabies epizootics in Colombia, 1994-2002: evidence of human and canine rabies associated with chiroptera. , Biomedica: Vol. 23 No. 1 (2003)
- María Belén Jaimes, Diana C. Cáceres, Fernando de la Hoz, Camilo Gutiérrez, Diana Herrera, Jairo Pinilla, Alexandra Porras, Fabio Rodríguez, Martha Velandia, Risk factors for severe acute lower respiratory tract infection in Bogota, 2001. , Biomedica: Vol. 23 No. 3 (2003)
- Oscar Eugenio Sierra, María Antonia Gaona de Hernández, Gloria Janneth Rey, Permeability to phi chi 174 bacteriophages in polyolephin membrane condoms. , Biomedica: Vol. 25 No. 4 (2005)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























