Identification of medication errors through a monitoring and minimization program in outpatients in Colombia, 2018-2019
Abstract
Introduction: The use of drugs may involve medication errors leading to hospitalization, increased costs related to care, and even death.
Objective: To determine the prevalence of medication errors reported in a pharmacovigilance information system in Colombia between 2018 and 2019.
Materials and methods: We conducted an observational study based on the records of medication errors from a pharmacovigilance system covering 8.5 million outpatients
affiliated with the Colombian health system. The errors were categorized from A (potential situations to error) to I (an error that could lead to death). We performed a descriptive analysis and established the prevalence of medication errors.
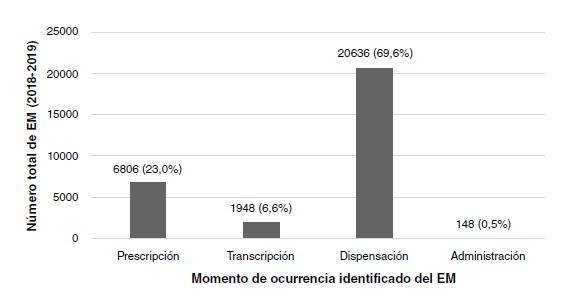
Results: During 2018 and 2019, 29,538 medication errors in outpatients were reported with a general prevalence of 1.93 per 10,000 drugs dispensed. The errors that reached the patient and caused damage (types E, F, and I) occurred in 0.02% (n=6) of the patients. Most of them were related to the dispensation (n=20,636; 69.9%) and the possible most common cause was the lack of concentration at the time of dispensing (n=9185; 31.1%). The pharmacological groups most involved in medication errors were antidiabetics (8.0%), renin-angiotensin system inhibitors (7.6%), and analgesics (6.0%).
Conclusions: Medication errors are relatively rare situations, generally classified as circumstances or events capable of generating the error (type A error). In low proportion, they can reach the patient and cause damage or even death.
Downloads
References
Benjamin DM. Reducing medication errors and increasing patient safety: case studies in clinical pharmacology. J Clin Pharmacol. 2003;43:768-83. https://doi.org/10.1177/0091270003254794
Goedecke T, Ord K, Newbould V, Brosch S, Arlett P. Medication errors: New EU good practice guide on risk minimization and error prevention. Drug Saf. 2016;39:491-500. https://doi.org/10.1007/s40264-016-0410-4
Institute of Medicine (US) Committee on Quality of Health Care in America, Kohn LT, Corrigan JM, Donaldson MS. To err is human: Building a safer health system. Washington, D.C.: National Academies Press; 2000. p. 26-155.
Bates DW, Singh H. Two decades since to err is human: An assessment of progress and emerging priorities in patient safety. Health Aff (Millwood). 2018;37:1736-43. https://doi.org/10.1377/hlthaff.2018.0738
Lewis PJ, Dornan T, Taylor D, Tully MP, Wass V, Ashcroft DM. Prevalence, incidence and nature of prescribing errors in hospital inpatients: A systematic review. Drug Saf. 2009;32:379-89. https://doi.org/10.2165/00002018-200932050-00002
Avery AJ, Ghaleb M, Barber N, Dean Franklin B, Armstrong SJ, Serumaga B, et al. The prevalence and nature of prescribing and monitoring errors in English general practice: A retrospective case note review. Br J Gen Pract. 2013;63:e543-53. https://doi.org/10.3399/bjgp13X670679
Machado-Alba JE, Ossa-Ochoa LM, Lotero-Jaramillo N, Valencia-Rojas A. Identificación de errores de medicación en un hospital de primer nivel de Pereira, Colombia. Revista de la Facultad de Medicina. 2013;61:267-73.
Machado-Alba JE, Moncada JC, Moreno-Gutiérrez PA. Medication errors in outpatient care in Colombia, 2005-2013. Biomédica. 2016;36:251-7. https://doi.org/10.7705/biomedica.v36i2.2693
Joolaee S, Hajibabaee F, Peyrovi H, Haghani H, Bahrani N. The relationship between incidence and report of medication errors and working conditions. Int Nurs Rev. 2011;58:37-44. https://doi.org/10.1111/j.1466-7657.2010.00872.x
National Coordinating Council for Medication Error Reporting and Prevention. Types of medication errors. Fecha de consulta: 6 de febrero de 2019. Disponible en: https://www.nccmerp.org/types-medication-errors
Machado-Alba JE, Moreno-Gutiérrez PA, Moncada-Escobar JC. Hospital medication errors in a pharmacovigilance system in Colombia. Farm Hosp. 2015;39:338-43. https://doi.org/10.7399/fh.2015.39.6.8899
Bjorksten KS, Bergqvist M, Andersen-Karlsson E, Benson L, Ulfvarson J. Medication errors as malpractice-a qualitative content analysis of 585 medication errors by nurses in Sweden. BMC Health Serv Res. 2016;16:431. https://doi.org/10.1186/s12913-016-1695-9
Zacher JM, Cunningham FE, Zhao X, Burk ML, Moore VR, Good CB, et al. Detection of potential look-alike/sound-alike medication errors using Veterans Affairs administrative databases. Am J Health Syst Pharm. 2018;75:1460-6. https://doi.org/10.2146/ajhp170703
Phatak HM, Cady PS, Heyneman CA, Culbertson VL. Retrospective detection of potential medication errors involving drugs with similar names. J Am Pharm Assoc (2003). 2005;45:616-21. https://doi.org/10.1331/1544345055001247
Otero MJ, Moreno-Gomez AM, Santos-Ramos B, Agra Y. Developing a list of high-alert medications for patients with chronic diseases. Eur J Intern Med. 2014;25:900-8. https://doi.org/10.1016/j.ejim.2014.10.021
Nicolas A, Eickhoff C, Griese N, Schulz M. Drug-related problems in prescribed medicines in Germany at the time of dispensing. Int J Clin Pharm. 2013;35:476-82. https://doi.org/10.1007/s11096-013-9769-9
Torres DR, Portilla A, Machado-Duque ME, Machado-Alba JE. Trends in the use and cost of human and analogue insulins in a Colombian population, 2011-2015. Public Health. 2017;153:64-9. https://doi.org/10.1016/j.puhe.2017.08.011
Rodziewicz TL, Hipskind JE. Medical error prevention. 2020. Treasure Island, FL: StatPearls Publishing; 2020.
Simas da Rocha B, Garcia Moraes C, Miyake Okumura L, da Cruz F, Sirtori L, da Silva Pons E. Interventions to reduce problems related to the readability and comprehensibility of drug packages and labels: A systematic review. J Patient Saf. 2020. https://doi.org/10.1097/PTS.0000000000000619
Invima. Resolución Nº 2004009455 2004. Fecha de consulta: 6 de julio de 2020. Disponible en: https://www.invima.gov.co/documents/20143/828720/resolucion_2004009455_2004.pdf/14bea02f-1fb0-79ed-767d-6c7bf6f18aaa
Some similar items:
- Jorge E. Machado-Alba, Juan Carlos Moncada, Paula Andrea Moreno-Gutiérrez, Medication errors in outpatient care in Colombia, 2005-2013 , Biomedica: Vol. 36 No. 2 (2016)
- Jorge Enrique Machado-Alba, Manuel José Londoño-Builes, Luis Felipe Echeverri-Cataño, Sergio Andrés Ochoa-Orozco, Adverse drug reactions in Colombian patients, 2007-2013: Analysis of population databases , Biomedica: Vol. 36 No. 1 (2016)
- Carlos Andrés Badillo, Lizeth Katherine Barrera, Gerson Arias, Gabriel Fernando Tribiño, Oscar Andrés Gamboa, Julio César García, Ana María Granada, Incidence of antiretroviral drug-related problems in the treatment of HIV among hospitalized patients in the Hospital Santa Clara, Bogotá , Biomedica: Vol. 39 No. 3 (2019)
- Omar Segura, Carlos Maldonado, Evaluation of medication side-effects from an economic perspective. , Biomedica: Vol. 23 No. 4 (2003)
- Jorge E. Machado-Alba, Luis Felipe Echeverri-Cataño, Manuel José Londoño-Builes, Paula Andrea Moreno-Gutiérrez, Sergio Andrés Ochoa-Orozco, Joaquín Octavio Ruiz-Villa, Social, cultural and economic factors associated with self-medication , Biomedica: Vol. 34 No. 4 (2014)
- Roxana De las Salas, Daniela Díaz-Agudelo, Adverse drug reactions in neonates hospitalized in neonatal intensive care units in Barranquilla, Colombia , Biomedica: Vol. 37 No. Sup.1 (2017): Suplemento 1, Alteraciones del sistema nervioso
- María Alejandra Montoya-Giraldo, Dayana Vanessa Montoya, David Alexander Atehortúa, Jefferson Antonio Buendía, Andrés Felipe Zuluaga, Myoclonus induced by salbutamol: A case report , Biomedica: Vol. 38 No. 3 (2018)
- Efraín Guillermo Sánchez , David Acosta , Juan Álvarez, Gabriela Sánchez , Julio García-Casallas, Disseminated cryptococcosis by biological therapy: We must manage the risk , Biomedica: Vol. 42 No. 2 (2022)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























