Impact of the allergen-specific immunotherapy in pediatric patients with asthma treated at a health institution in Colombia
Abstract
Introduction: Asthma is a chronic and potentially serious disease and 80% of the cases have an allergic etiology. In this sense, allergen-specific immunotherapy is an alternative that modulates the natural course of the disease.
Objective: To evaluate the impact of immunotherapy in pediatric asthma patients treated at a health institution in Colombia.
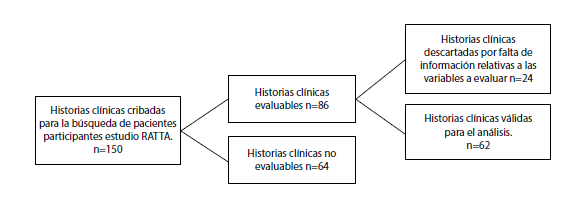
Materials and methods: We conducted an observational descriptive study with an analytical cross-sectional component. Sixty-two patients diagnosed with allergic asthma sensitized to dust mites and treated with at least 6 doses of mite immunotherapy were included. We assessed the impact of immunotherapy using the Asthma Control Test (ACT), the Global Initiative for Asthma (GINA) treatment scale, and spirometry values.
Results: The ACT score before the start reported 30% of patients with uncontrolled asthma, 28% with good control, and 4% with totally controlled asthma. Of the patients with uncontrolled asthma, 46.7% achieved good control and 23.3% total control. Regarding patients’ perception of improvement with the immunotherapy, 9.75% perceived a response of less than 50%, 45.2% one between 50% -90%, and 41.9% reported response equal to or greater than 90%. No significant changes in FEV1 values were found in spirometry.
Conclusions: Significant changes in the ACT scores and the perception of disease improvement were observed in the population evaluated with specific mite immunotherapy, i.e., it had a positive impact on the natural course of the disease.
Downloads
References
Russell RJ, Brightling C. Pathogenesis of asthma: Implications for precision medicine. Clin Sci. 2017;131:1723-35. https://doi.org/10.1042/CS20160253
Dennis R, Caraballo L, García E, Caballero A, Aristizábal G, Córdoba H, et al. Asthma and other allergic conditions in Colombia: A study in 6 cities. Ann Allergy Asthma Immunol. 2004;93:568-74. https://doi.org/10.1016/S1081-1206(10)61265-3
Dennis RJ, Caraballo L, García E, Rojas MX, Rondón MA, Pérez A, et al. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: A cross-sectional study. BMC Pulm Med. 2012;12:17. https://doi.org/10.1186/1471-2466-12-17
Global Initiative for Asthma. Pocket guide for asthma management: For adults and children over 5 years. GINA; 2019. Fecha de consulta: 26 de noviembre de 2019. Disponible en: https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf
Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: Document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926-38. https://doi.org/10.1016/j.jaci.2010.07.019
Tran TN, Zeiger RS, Peters SP, Colice G, Newbold P, Goldman M, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116:37-42. https://doi.org/10.1016/j.anai.2015.10.027
Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355-60. https://doi.org/10.1016/j.jaci.2010.11.037
Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139: 541-9.e8. https://doi.org/10.1016/j.jaci.2016.08.014
Durham SR. Allergen immunotherapy: 100 years on. Clin Exp Allergy. 2011;41:1171. https://doi.org/10.1111/j.1365-2222.2009.03843.x
Yepes-Núñez JJ, Gómez C, Espinoza Y, Cardona R. Impacto de la inmunoterapia subcutánea con Dermatophagoides farinae y Dermatophagoides pteronyssinus sobre la calidad de vida de pacientes con rinitis y asma alérgica. Biomédica. 2014;34:282-90. https://doi.org/10.7705/biomedica.v34i2.1744
Gaviria R, Ocampo J, Londoño J, Calvo V, Cardona R, Sánchez J. Sensibilización IgE y las condiciones sociodemográficas como determinantes en la gravedad del asma. Revista Alergia México. 2017;64:439. https://doi.org/10.29262/ram.v64i4.291
Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42:1012-4. https://doi.org/10.1093/ije/dys223
Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549-56. https://doi.org/10.1016/j.jaci.2006.01.011
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl.86):8-160. https://doi.org/10.1111/j.1398-9995.2007.01620.x
Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018;73:765-98. https://doi.org/10.1111/all.13317
Gandhi VD, Davidson C, Asaduzzaman M, Nahirney D, Vliagoftis H. House dust mite interactions with airway epithelium: Role in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13:262-70. https://doi.org/10.1007/s11882-013-0349-9
Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010. Cochrane Database Syst Rev. 2010;CD001186. https://doi.org/10.1002/14651858.cd001186.pub2
Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy. 2017;72:1825-48. https://doi.org/10.1111/all.13208
Acevedo N, Zakzuk J, Caraballo L. House dust mite allergy under changing environments. Allergy Asthma Immunol Res. 2019;11:450-69. https://doi.org/10.4168/aair.2019.11.4.450
Sánchez-Caraballo J, Diez-Zuluaga S, Cardona-Villa R. Sensitization to aeroallergens in allergic patients from Medellín, Colombia. Rev Alerg Mex. 2012;59:139-47.
Fernández-Caldas E, Puerta L, Mercado D, Lockey RF, Caraballo LR. Mite fauna, Der pI, Der f I and Blomia tropicalis allergen levels in a tropical environment. Clin Exp Allergy. 1993;23:292-7. https://doi.org/10.1111/j.1365-2222.1993.tb00325.x
Some similar items:
- Juan José Yepes-Núñez, Carolina Gómez, Yeinis Espinoza, Ricardo Cardona, The impact of subcutaneous immunotherapy with Dermatophagoides farinae and Dermatophagoides pteronyssinus on the quality of life of patients with allergic rhinitis and asthma , Biomedica: Vol. 34 No. 2 (2014)
- Jorge Sánchez, Ricardo Cardona, Luis Caraballo, Carlos Serrano, Ruth Ramírez, Susana Díez, Elizabeth García, Ana María Segura, Alfonso Cepeda, María Minotas, Allergen immunotherapy: Mechanisms of action, and therapeutic and socioeconomic impact Consensus of the Asociación Colombiana de Alergia, Asma e Imunología , Biomedica: Vol. 36 No. 3 (2016)
- Ángela M. Pedraza, Carlos E. Rodríguez-Martínez, Ranniery Acuña, Initial validation of a scale to measure the burden for parents/caregivers of children with asthma and factors associated with this burden in a population of asthmatic children , Biomedica: Vol. 33 No. 3 (2013)
- Ricardo Cardona, Ruth Helena Ramírez, Zulma Reina, Mauricio Fernando Escobar, Edison Morales, Allergy and intolerance to nonsteroidal antinflammatory drugs: successful desensitization in three cases , Biomedica: Vol. 29 No. 2 (2009)
- Juan Carlos Hernández, Carlos Julio Montoya, Silvio Urcuqui-Inchima, The role of toll-like receptors in viral infections: HIV-1 as a model , Biomedica: Vol. 27 No. 2 (2007)
- José Fernando Cantillo, Leonardo Puerta, New approaches for allergen-specific immunotherapy , Biomedica: Vol. 30 No. 3 (2010)
- Astrid Berena Herrera, Laura A. Rodríguez, Jürg Niederbacher, Biological pollution and its relationship with respiratory symptoms indicative of asthma, Bucaramanga, Colombia , Biomedica: Vol. 31 No. 3 (2011)
- Marcela González, Claudia Patricia González, Alvaro Sanabria, Ultrasonographic estimation of the normal volume of the thyroid gland in pediatric populations. , Biomedica: Vol. 26 No. 1 (2006)
- Jorge Sánchez, María Nelly Restrepo, José Mopan, Carlos Chinchilla, Ricardo Cardona, Milk and egg allergy: Diagnosis, management and implications for Latin America , Biomedica: Vol. 34 No. 1 (2014)
- Rodrigo Sarmiento, Luis Jorge Hernández, Edna Katalina Medina, Natalia Rodríguez, Jesús Reyes, Respiratory symptoms associated with air pollution in five localities of Bogotá, 2008-2011, a dynamic cohort study , Biomedica: Vol. 35 (2015): Agosto, Suplemento 2, Salud y contaminantes ambientales
Funding data
-
Universidad de Antioquia
Grant numbers 20730003
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























