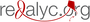Efficacy of tafenoquine in the prophylaxis and treatment of malaria by Plasmodium vivax, systematic review and meta-analysis
Abstract
Introduction: Tafenoquine was approved in 2018 by the Food and Drug Administration in the United States and in 2019 by the Therapeutic Goods Administration in Australia. Its administration in a single dose and its mechanism of action in the acute and latent phases of the disease have been studied to change the treatment regimen for Plasmodium vivax malaria.
Objective: To evaluate the available scientific evidence of the efficacy of tafenoquine in prophylaxis and treatment between 2009 and 2019.
Materials and methods: We established the MeSH and DeCS descriptors and we used the syntax ((Malaria Vivax) AND (tafenoquine) AND (prophylaxis)) OR [(Malaria Vivax) AND (tafenoquine) AND (relapse)] in the following databases: Pubmed, The Cochrane Central Register of Controlled Clinical Trials (CENTRAL), ISIS Web of Science, Lilacs, and Scopus. The results obtained were subjected to critical analysis (CASPE matrix). The quantitative analysis was performed with risk differences in survival analysis (Kaplan Meier) in the final three articles.
Results: Three studies underwent meta-analysis (Llanos-Cuentas, 2014; Llanos-Cuentas, 2019, and Lacerda, 2019) to evaluate the efficacy of the treatment with tafenoquine compared to primaquine. A global risk difference of 0.04 was obtained (95% CI: 0.00-0.08; p=0.07). Tafenoquine did not show inferiority in the efficacy of treatment compared to the primaquine scheme.
Conclusion: Tafenoquine is a therapeutic alternative to primaquine that improves adherence, which could bring Colombia closer to the goals of the World Technical Strategy against Malaria 2016-2030.
Downloads
References
World Health Organization. World Malaria Report: 20 years of global progress and challenges. 2020. Fecha de consulta: 17 de noviembre de 2020. Disponible en: https://www.who.int/publications/i/item/9789240015791
Pan American Health Organization. Epidemiological Update - Malaria in the Americas Situation. 2019. Fecha de consulta: 31 de octubre de 2020. Disponible en: https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=2019-2&alias=51009-18-november-2019-malaria-epidemiological-update&Itemid=270&lang=en
Instituto Nacional de Salud. Boletín epidemiológico, semana 53 de 2020: Malaria. Fecha de consulta: 17 de noviembre de 2020. Disponible en: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2020_Boletin_epidemiologico_semana_53.pdf
Instituto Nacional de Salud. Boletín epidemiológico, semana 52 de 2019: Malaria. Fecha de consulta: 17 de noviembre de 2020. Disponible en: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019_Boletin_epidemiologico_semana_52.pdf
Instituto Nacional de Salud. Boletín epidemiológico, semana 52 de 2018: Malaria. Fecha de consulta: 17 de noviembre de 2020. Disponible en: https://www.ins.gov.co/buscadoreventos/BoletinEpidemiologico/2018%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2052.pdf
Organización Mundial de la Salud. Estrategia técnica mundial contra la malaria 2016 - 2030. Fecha de consulta: 20 de noviembre de 2020. Disponible en: http://apps.who.int/iris/bitstream/10665/186671/1/9789243564999_spa.pdf
Instituto Nacional de salud. Guía para la atención integral del paciente con malaria. 2010. Fecha de consulta: 10 de noviembre de 2020. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/Guia-atencion-clinica-malaria-2011.pdf
Australian Government Department of Health. AusPAR: Tafenoquine succinate - Therapeutic Goods Administration (TGA). Fecha de consulta: 17 de noviembre 2020. Disponible en: https://www.tga.gov.au/auspar/auspar-tafenoquine-succinate-0
Mayence A, Vanden JJ. Tafenoquine: A 2018 novel FDA-approved prodrug for the radical cure of Plasmodium vivax malaria and prophylaxis of malaria. Pharmaceuticals. 2019;12:115. https://doi.org/10.3390/ph12030115
Llanos-Cuentas A, Lacerda MVG, Hien TT, Vélez ID, Namaik-larp C, Chu CS, et al. Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019;380:229-41. https://doi.org/10.1056/NEJMoa1802537
Ebstie YA, Abay SM, Tadesse WT, Ejigu DA. Tafenoquine and its potential in the treatment and relapse prevention of Plasmodium vivax malaria: The evidence to date. Drug Des Devel Ther. 2016;10:2387-99. https://doi.org/10.2147/DDDT.S61443
Llanos-Cuentas A, Lacerda MV., Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): A multicentre, double-blind, randomised, phase 2b doseselection study. Lancet. 2014;383:1049-58. https://doi.org/10.1016/S0140-6736(13)62568-4
National Center for Biotechnology Information. Malaria, Vivax – MeSH. Fecha de consulta: 7 de noviembre de 2020. Disponible en: https://www.ncbi.nlm.nih.gov/mesh/?term=malaria+vivax
National Center for Biotechnology Information. Tafenoquine – MeSH. Fecha de consulta: 7 noviembre 2020. Disponible en: https://www.ncbi.nlm.nih.gov/mesh/?term=tafenoquine
National Center for Biotechnology Information. Pre-exposure prophylaxis -MeSH. Fecha de consulta: 7 de noviembre de 2020. Disponible en: https://www.ncbi.nlm.nih.gov/mesh/68065129
National Center for Biotechnology Information. Recurrence – MeSH. Fecha de consulta: 7 de noviembre de 2020. Disponible en: https://www.ncbi.nlm.nih.gov/mesh/68012008
Critical Appraisal Skills Programme. Instrumentos para la lectura crítica – CASPe. Fecha de consulta: 16 de noviembre de 2020. Disponible en: http://www.redcaspe.org/herramientas/instrumentos
Nasveld PE, Edstein MD, Reid M, Brennan L, Harris IE, Kitchener SJ, et al. Randomized, double-blind study of the safety, tolerability, and efficacy of tafenoquine versus mefloquine for malaria prophylaxis in nonimmune subjects. Antimicrob Agents Chemother. 2010;54:792-8. https://doi.org/10.1128/AAC.00354-09
Rodrigo C, Rajapakse S, Fernando SD. Tafenoquine for primary and terminal prophylaxis of malaria in apparently healthy people: A systematic review. Trans R Soc Trop Med Hyg. 2019 ;113 :579-86. https://doi.org/10.1093/trstmh/trz052
Lacerda MVG, Llanos-Cuentas A, Krudsood S, Lon C, Saunders DL, Mohammed R, et al. Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019 ;380 :215-28. https ://doi.org/10.1056/NEJMoa1710775
Fukuda MM, Krudsood S, Mohamed K, Green JA, Warrasak S, Noedl H, et al. A randomized, double-blind, active-control trial to evaluate the efficacy and safety of a three-day course of tafenoquine monotherapy for the treatment of Plasmodium vivax malaria. PloS One. 2017;12:e0187376. https://doi.org/10.1371/journal.pone.0187376
Rajapakse S, Rodrigo C, Fernando SD. Tafenoquine for preventing relapse in people with Plasmodium vivax malaria. Cochrane Database Syst Rev. 2015;CD010458. https://doi.org/10.1002/14651858.CD010458.pub2
Beck HP, Wampfler R, Carter N, Koh G, Osorio L, Rueangweerayut R, et al. Estimation of the antirelapse efficacy of tafenoquine, using Plasmodium vivax genotyping. J Infect Dis. 2016;213:794-9. https://doi.org/10.1093/infdis/jiv508
Rueangweerayut R, Bancone G, Harrell EJ, Beelen AP, Kongpatanakul S, Möhrle JJ, et al. Hemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6- phosphate dehydrogenase (G6PD) deficiency (G6PD Mahidol Variant) versus G6PD-normal volunteers. Am J Trop Med Hyg. 2017:97:702-11. https://doi.org/10.4269/ajtmh.16-0779
Uribe A. Deficiencia de glucosa 6-fosfato deshidrogenasa en Colombia: memorias de 22 años de tamizaje de alto riesgo. Revista Med, 2017;25:7-21. https://doi.org/10.18359/rmed.3206
Fonseca J, Álvarez A, Ríos A, Vásquez MF. Deficiencia de glucosa 6-fostato deshidrogenasa en hombres sanos y en pacientes maláricos, Turbo (Antioquia, Colombia). Rev Bras Epidemiol. 2008;11:252-65. https://doi.org/10.1590/S1415-790X2008000200007
World Health Organization. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria. Global Malaria Programme. Geneva: WHO; 2016.
Some similar items:
- Ana María Vásquez, Felipe Sanín, Luis Gonzalo Álvarez, Alberto Tobón, Alexandra Ríos, Silvia Blair, Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development , Biomedica: Vol. 29 No. 2 (2009)
- Jaime Carmona-Fonseca, Gonzalo Álvarez, Silvia Blair, Plasmodium vivax malaria: treatment of primary attacks with primaquine, in three different doses, and a fixed dose of chloroquine, Antioquia, Colombia, 2003-2004 , Biomedica: Vol. 26 No. 3 (2006)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |