Facial nerve injury-associated hippocampal microglial activation
Abstract
Introduction: Facial nerve injury induces changes in hippocampal long-term synaptic plasticity and affects both object recognition memory and spatial memory consolidation (i.e., hippocampus-dependent tasks). Although facial nerve injury-associated microglial activation has been described regarding the primary motor cortex, it has not been ascertained whether something similar occurs in the hippocampus. Peripheral nerve injuryassociated microglial changes in hippocampal tissue could explain neuronal changes in the contralateral hippocampus.
Objective: To characterize the effect of unilateral facial nerve injury on microglial proliferation and activation in the contralateral hippocampus.
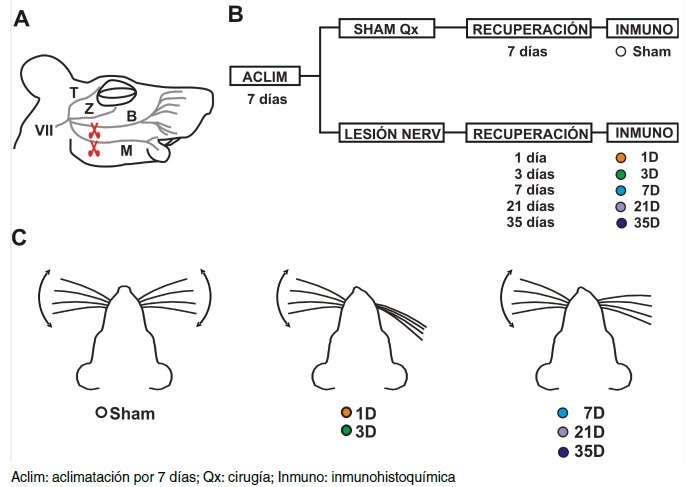
Materials and methods. Immunohistochemical experiments detected microglial cells in the hippocampal tissue of rats that had undergone facial nerve injury. The animals were sacrificed at specific times after injury to evaluate hippocampal microglial cell proliferation (cell density) and activation (cell area); sham-operated animals were compared to lesioned animals sacrificed 1, 3, 7, 21, or 35 days after injury.
Results: Facial nerve-injured rats’ hippocampal microglial cells proliferated and adopted an activated phenotype 3- to 21-days post-lesion. Such modifications were transient since the microglial cells returned to their resting state five weeks after injury, despite the injury’s irreversible nature.
Conclusions: Facial nerve injury causes the transient proliferation and activation of microglial cells in the hippocampus. This finding might partly explain the morphological and electrophysiological changes described for CA1 pyramidal neurons and the impairment of spatial memory consolidation which has previously been observed in facial nerve-injured rats.
Downloads
References
Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154-78. https://doi.org/10.1016/j.brainresrev.2003.11.004
Múnera A, Cuestas DM, Troncoso J. Peripheral facial nerve lesions induce changes in the firing properties of primary motor cortex layer 5 pyramidal cells. Neuroscience. 2012;223:140-51. https://doi.org/10.1016/j.neuroscience.2012.07.063
Urrego D, Múnera A, Troncoso J. Retracción a largo plazo del árbol dendrítico de neuronas piramidales córtico-faciales por lesiones periféricas del nervio facial. Biomédica. 2011;31:560-9. https://doi.org/10.7705/biomedica.v31i4.440
Urrego D, Troncoso J, Múnera A. Layer 5 pyramidal neurons’ dendritic remodeling and increased microglial density in primary motor cortex in a murine model of facial paralysis. Biomed Res Int. 2015;2015:482023. https://doi.org/10.1155/2015/482023
Torrado-Arévalo R, Troncoso J, Múnera A. Facial nerve axotomy induces changes on hippocampal CA3-to-CA1 long-term synaptic plasticity. Neuroscience. 2021;475:197-205. https://doi.org/10.1016/j.neuroscience.2021.08.023
Moreno C, Vivas O, Lamprea NP, Lamprea MR, Múnera A, Troncoso J. Vibrissal paralysis unveils a preference for textural rather than positional novelty in the one-trial object recognition task in rats. Behav Brain Res. 2010;211:229-35. https://doi.org/10.1016/j.bbr.2010.03.044
Patarroyo WE, García-Pérez M, Lamprea M, Múnera A, Troncoso J. Vibrissal paralysis produces increased corticosterone levels and impairment of spatial memory retrieval. Behav Brain Res. 2017;320:58-66. https://doi.org/10.1016/j.bbr.2016.11.045
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314-8. https://doi.org/10.1126/science.1110647
Dávalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752-8. https://doi.org/10.1038/nn1472
Ulland TK, Wang Y, Colonna M. Regulation of microglial survival and proliferation in health and diseases. Semin Immunol. 2015;27:410-5. https://doi.org/10.1016/j.smim.2016.03.011
Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441-68. https://doi.org/10.1146/annurev-immunol-051116-052358
Tremblay M-È, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLOS Biol. 2010;8:e1000527. https://doi.org/10.1371/journal.pbio.1000527
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974-80. https://doi.org/10.1523/JNEUROSCI.4363-08.2009_15
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456-8. https://doi.org/10.1126/science.1202529
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691-705. https://doi.org/10.1016/j.neuron.2012.03.026
Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058-68. https://doi.org/10.1016/j.bbi.2010.02.001
Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013;23:1784-97. https://doi.org/10.1016/j.bbi.2010.02.001
Jáuregui-Huerta F, Ruvalcaba-Delgadillo Y, González-Castañeda R, García-Estrada J, González-Pérez O, Luquin S. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. 2010;6:195-204. https://doi.org/10.2174/157339510791823790
Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262-76. https://doi.org/10.2174/13894501113149990208
Paxinos G, Watson C. The rat brain in stereotaxic coordinates-The New Coronal Set. 6th edition. London, UK: Academic Press; 2007.
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1-9. https://doi.org/10.1016/s0169-328x(98)00040-0
Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62:282-97. https://doi.org/10.1016/0014-4886(78)90058-4
Schmajuk NA. Role of the hippocampus in temporal and spatial navigation: An adaptive neural network. Behav Brain Res. 1990;39:205-29. https://doi.org/10.1016/0166-4328(90)90028-d
Gulli RA, Duong LR, Corrigan BW, Doucet G, Williams S, Fusi S, et al. Context-dependent representations of objects and space in the primate hippocampus during virtual navigation.Nat Neurosci. 2020;23:103-12. https://doi.org/10.1038/s41593-019-0548-3
Bird CM. The role of the hippocampus in recognition memory. Cortex. 2017;93:155-65. https://doi.org/10.1016/j.cortex.2017.05.016
Hartmann MJ, Johnson NJ, Towal RB, Assad C. Mechanical characteristics of rat vibrissae: Resonant frequencies and damping in isolated whiskers and in the awake behaving animal. J Neurosci. 2003;2:6510-9. https://doi.org/10.1523/JNEUROSCI.23-16-06510.2003
Bellistri E, Aguilar J, Brotons-Mas JR, Foffani G, Prida LM de la. Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J Physiol. 2013;591:2667-86. https://doi.org/10.1113/jphysiol.2013.251892
Ren W-J, Liu Y, Zhou L-J, Li W, Zhong Y, Pang R-P, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979-92. https://doi.org/10.1038/npp.2010.236
Fasick V, Spengler RN, Samankan S, Nader ND, Ignatowski TA. The hippocampus and TNF: Common links between chronic pain and depression. Neurosci Biobehav Rev. 2015;53:139-59. https://doi.org/10.1016/j.neubiorev.2015.03.014
Fiore NT, Austin PJ. Peripheral nerve injury triggers neuroinflammation in the medial prefrontal cortex and ventral hippocampus in a subgroup of rats with coincident affective behavioural changes. Neuroscience. 2019;416:147-67. https://doi.org/10.1016/j.neuroscience.2019.08.005
Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immun. 2016;56:397-411. https://doi.org/10.1016/j.bbi.2016.04.012
Liu Y, Zhou L-J, Wang J, Li D, Ren W-J, Peng J, et al. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci. 2017;37:871-81. https://doi.org/10.1523/JNEUROSCI.2235-16.2016
McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115-42. https://doi.org/10.1002/cne.20342
Vertes RP, Hoover WB, Valle ACD, Sherman A, Rodríguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768-96. https://doi.org/10.1002/cne.21135
Wang Y-L, Han Q-Q, Gong W-Q, Pan D-H, Wang L-Z, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. 2018;15:21. https://doi.org/10.1186/s12974-018-1054-3
Some similar items:
- Diana Urrego, Alejandro Múnera, Julieta Troncoso, Peripheral facial nerve lesion induced long-term dendritic retraction in pyramidal cortico-facial neurons , Biomedica: Vol. 31 No. 4 (2011)
- Edwin Abraham Medina, Middle ear adenoma , Biomedica: Vol. 29 No. 3 (2009)
- Elpidia Poveda, Pilar Trujillo, Francisco Ruiz, Elizabeth Lopez, Glucose and insulin levels in Wistar rats submitted to high fat diet and treatment with mimetic leptin peptides , Biomedica: Vol. 28 No. 1 (2008)
- Aura Caterine Rengifo, Orlando Torres-Fernández, Decreased number neurons expressing GABA in the cerebral cortex of rabies-infected mice , Biomedica: Vol. 27 No. 4 (2007)
- Mario Francisco Guerrero, Elements for the effective evaluation of natural products with possible antihypertensive effects , Biomedica: Vol. 29 No. 4 (2009)
- Lauro Figueroa, Francisco Díaz, Avelardo Camacho, Eliseo Díaz, Rolando Marvin, Activity induced by androsterone and hemisuccinate of androsterone on perfusion pressure and vascular resistance , Biomedica: Vol. 29 No. 4 (2009)
- Biviana Andrea Duque, Diego Aranzazu, Piedad Agudelo-Flórez, Andrés F. Londoño, Víctor H. Quiroz, Juan David Rodas, Rattus norvegicus as an indicator of circulation of Capillaria hepatica and Taenia taeniaeformis on a groceries trade center of Medellín, Colombia , Biomedica: Vol. 32 No. 4 (2012)
- Rafael A. Ulloque, Effects of cocaine on y-aminobutyrate, glutamate and aspartate levels in rat nucleus accumbens and hippocampus , Biomedica: Vol. 21 No. 3 (2001)
- Elpidia Poveda, Paola Ayala, Milena Rodríguez, Edgar Ordóñez, Cesar Baracaldo, Willman Delgado, Martha Guerra, Effects of vegetal oil supplementation on the lipid profile of Wistar rats . , Biomedica: Vol. 25 No. 1 (2005)
- Silvana Marisa Montenegro, María Cristina Tarrés, Juan Carlos Picena, Stella Maris Martínez, Feeding behavior and glycemic profile in two lines of rats with genetic diabetes. , Biomedica: Vol. 25 No. 4 (2005)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























