Natural SARS-CoV- 2 infection in domestic cats and dogs of humans diagnosed with COVID-19 in Valle de Aburrá, Antioquia
Abstract
Introduction: The severe acute respiratory syndrome caused by the new coronavirus SARS-CoV-2 is the cause of the health emergency due to the COVID-19 pandemic.
Although humans are the main susceptible host, experimental studies and reported cases of natural infection have evidenced scenarios of SARS-CoV-2 reverse zoonosis in animals.
Objective: To evaluate the natural infection of SARS-CoV-2 in cats and dogs with owners diagnosed with COVID-19 in the Valle de Aburrá subregion in Antioquia, Colombia.
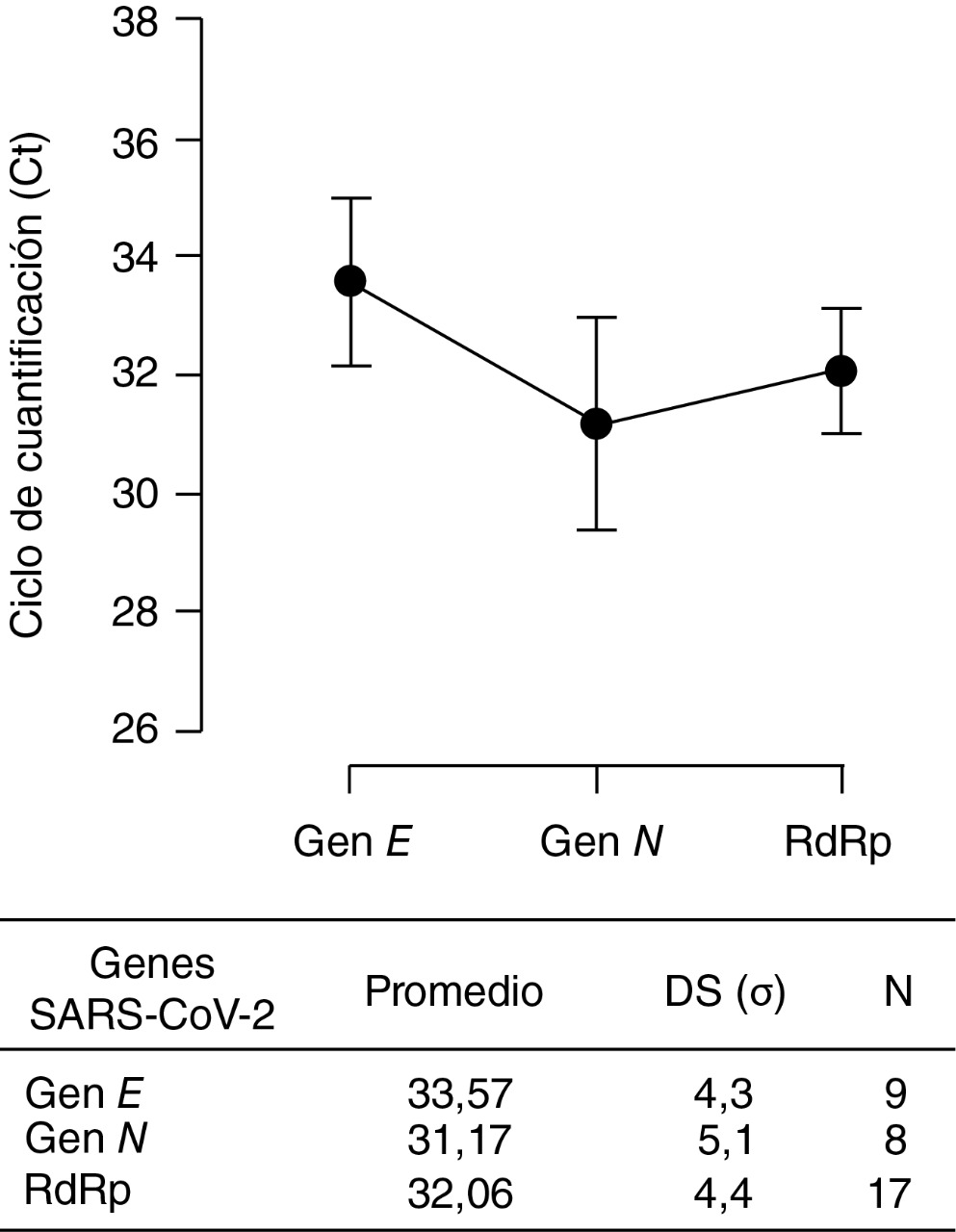
Materials and methods. The circulation of SARS-CoV-2 was evaluated by RT-qPCR and RT-PCR in samples of nasopharyngeal and oropharyngeal smears from cats and dogs whose owners presented latent COVID-19 infection. Positive cases were verified through amplification of N, E and RdRp gene fragments; with the latter being sequenced and the phylogenetically analyzed
Results. From 80 tested animals, 6 cats and 3 dogs resulted positive for natural SARSCoV-2 infection. These animals did not show any clinical signs; and their infected owners only reported mild signs of COVID-19, without clinical complications. Regarding analysis of one of the sequences, a single nucleotide polymorphism (SNP) was found, with a substitution in position 647, resulting in the change of the amino acid serine (S) for isoleucine (I). The cases occurred in the municipalities of Caldas, Medellín and Envigado.
Conclusions. It is inferred that natural infection in cats and dogs is associated with direct contact with a positive COVID-19 patient.
Downloads
References
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-92. https://doi.org/10.1038/s41579-018-0118-9
Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020;94:44-8. https://doi.org/10.1016/j.ijid.2020.03.004
Instituto Nacional de Salud. COVID-19 en Colombia. Fecha de consulta: 22 de octubre de 2021. Disponible en: https://www.ins.gov.co/Noticias/paginas/coronavirus.aspx
Global Change Data Lab. Statistics and Research Coronavirus Pandemic (COVID-19). Fecha de consulta: 22 de octubre de 2021. Disponible en: https://ourworldindata.org/coronavirus
Leroy EM, Ar Gouilh M, Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020 10:100133. https://doi.org/10.1016/j.onehlt.2020.100133
Organización Mundial de Sanidad Animal (OIE). Sistema Mundial de Información Zoosanitaria (OIE-WAHIS). Fecha de consulta: 22 de octubre de 2022. Disponible en: https://wahis.oie.int/#/home
Bonilla-Aldana DK, García-Barco A, Jiménez-Díaz SD, Bonilla-Aldana JL, Cardona-Trujillo MC, Muñoz-Lara F, et al. SARS-CoV-2 natural infection in animals: A systematic review of studies and case reports and series. Vet Q . 2021;41:250-67. https://doi.org/10.1080/01652176.2021.197028
Rivero R, Garay E, Serrano-Coll H, Ramírez JD, Martínez-Bravo C, Mattar S, et al. Humanto-dog transmission of SARS-CoV-2 lota variant: Should COVID-19 patients avoid close contact with their pets during illness? Sci Rep. 2021;1-15. https://doi.org/10.21203/rs.3.rs-821033/v1
Wang H, Wang F, Wang H, Zhao Q. Potential infectious risk from the pets carrying SARSCoV-2. Travel Med Infect Dis. 2020;35:101737. https://doi.org/10.1016/j.tmaid.2020.101737
Boklund A, Hammer AS, Quaade ML, Rasmussen TB, Lohse L, Strandbygaard B, et al. SARS-CoV-2 in Danish mink farms: Course of the epidemic and a descriptive analysis of the outbreaks in 2020. Animals (Basel). 2021;11:164. https://doi.org/10.3390/ani11010164
Departamento Administrativo Nacional de Estadística DANE. Censo Nacional de Población y Vivienda 2018 Colombia. Fecha de consulta: 20 de octubre de 2021. Disponible en: https://www.dane.gov.co/index.php/estadisticas
Ministerio de Salud y Protección Social. Lineamientos para el uso de pruebas diagnósticas de laboratorio durante la pandemia del SARS-CoV-2 (Covid-19) en Colombia. Fecha de consulta: 20 de octubre de 2021 .Disponible en : https://www.minsalud.gov.co/Ministerio/Institucional/Procesos y procedimientos/GIPS21.pdf
Ministerio de Salud y Protección Social. Orientaciones para el uso adecuado de los elementos de protección personal por parte de los trabajadores de la salud expuestos a COVID-19 en el trabajo y en su domicilio. Fecha de consulta: 20 de junio de 2021. Disponible en: https://www.minsalud.gov.co/Ministerio/Institucional/Procesos y procedimientos/GIPS20.pdf
Shi J, Wen Z, Zhong G, Yang H, Wang C, Liu R, et al. Susceptibility of ferrets, cats, dogs, and different domestic animals to SARS-coronavirus-2. Science. 2020;368:1016-20. https://doi.org/10.1126/science.abb7015
World Organisation for Animal Health. Considerations for sampling, testing, and reporting of SARS-CoV-2 in animals. Fecha de consulta: 20 junio de 2021. Disponible en: https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/COV-19/Sampling_Testing_and_Reporting_of_SARS-CoV-2_in_animals_final_7May_2020.pdf
Attelia-Dawn H. Diagnostic testing for COVID-19 bridging study for QIAamp viral RNA extraction vs Beckman RNAdvance vs Thermofisher MagMAX. Oak Ridge: U.S. Department of Energy; 2021. https://doi.org/10.2172/1766984
Kaya H, Çalışkan A, Okul M, Sarı T, Akbudak İH. Detection of SARS-CoV-2 in the tears and conjunctival secretions of Coronavirus disease 2019 patients. J Infect Dev Ctries. 2020;14:977-81. https://doi.org/10.3855/jidc.13224
Peleg O, Baneth G, Eyal O, Inbar J, Harrus S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Veterinary Parasitology. 2010;173:292-9. https://doi.org/10.1016/j.vetpar.2010.06.039
Yamashita-Kawanishi N, Sawanobori R, Matsumiya K, Uema A, Chambers JK, Uchida K, et al. Detection of Felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. J Vet Med Sci. 2018;80:1236-40. https://doi.org/10.1292/jvms.18-0089
Hall T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium 1999;40:95-8.
Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol Biol Evol. 2021;38:3022-7. https://doi.org/10.1093/molbev/msab120
Trifinopoulos J, Nguyen L, von Haeseler A, Minh B. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucl Acids Res. 2016;44:W232-5. https://doi.org/10.1093/nar/gkw256
Letunic I, Bork P. Interactive Tree of Life iTOL v5: An online tool for phylogenetic tree display and annotation. Nucl Acids Res. 2021;49:W293-6. https://doi.org/10.1093/nar/gkab301
Muñoz M, Patiño LH, Ballesteros N, Castañeda S, Luna N, Delgado L, et al. Striking lineage diversity of severe acute respiratory syndrome coronavirus 2 from non-human sources. One Health. 2022;14:100363. https://doi.org/10.1016/j.onehlt.2021.100363
Calvet GA, Pereira SA, Ogrzewalska M, Pauvolid-Corrêa A, Resende PC, Tassinari W de S, et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE. 2021;16:e0250853. https://doi.org/10.1371/journal.pone.0250853
Fuentealba NA, Moré G, Bravi ME, Unzaga JM, De Felice L, Salina M, et al. First detection and molecular analysis of SARS-CoV-2 from a naturally infected cat from Argentina. Vet Microbiol. 2021;260:109179. https://doi.org/10.1016/j.vetmic.2021.109179
Li R, Qiao S, Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect. 2020;80:469-96. https://doi.org/10.1016/j.jinf.2020.02.013
Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. https://doi.org/10.15252/embj.20105114
Damas J, Hughes G, Keough K, Painter C, Persky N, Corbo M, et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci U S A. 2020;117:22311-22. https://doi.org/10.1073/pnas.2010146117
Rendon-Marín S, Martínez-Gutiérrez M, Whittaker GR, Jaimes JA, Ruiz-Saenz J. SARS CoV-2 spike protein in silico interaction with ACE2 receptors from wild and domestic species. Front Genet. 2021;12:571707. https://doi.org/10.3389/fgene.2021.571707
Bonilla-Aldana K, Ruiz-Sáenz J, Martínez-Gutiérrez M, Tiwari R, Dhama K, Jaimes J, et al. Concerns on the emerging research of SARS-CoV-2 on felines: Could they be significant hosts/reservoirs? J Pure Appl Microbiol. 2020;14(Suppl.1):703-8. https://doi.org/10.22207/JPAM.14.SPL1.04
Leroy EM, Ar Gouilh M, Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a One-Health strategy to control the COVID-19 pandemic. One Health. 2020;10:100133. https://doi.org/10.1016/j.onehlt.2020.100133
Ministerio de Salud y Protección Social. Cobertura nacional de vacunación antirrábica de perros y gatos por departamento año 2018. Fecha de consulta 20 de junio de 2021. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/SA/coberturas-vacunacion-antirrabica-perros-gatos-2018.zip
Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-8. https://doi.org/10.1016/j.jare.2020.03.005
Maestre JP, Jarma D, Yu JRF, Siegel JA, Horner SD, Kinney KA. Distribution of SARSCoV-2 RNA signal in a home with COVID-19 positive occupants. Sci Total Environ. 2021;778:146201. https://doi.org/10.1016/j.scitotenv.2021.146201
Shervani Z, Khan I, Siddiqui NY, Khan T, Qazi UY. Risk of SARS-CoV-2 transmission from humans to pets and viceversa. Eur J Med Health Sci. 2021;3:34-8. https://doi.org/10.24018/ejmed.2021.3.1.684
Abdel-Moneim AS, Abdelwhab EM. Evidence for SARS-COV-2 infection of animal hosts. Pathogens. 2020;9:529. https://doi.org/10.3390/pathogens9070529
Barroso-Arévalo S, Sánchez-Morales L, Barasona JA, Rivera B, Sánchez R, Risalde MA, et al. Evaluation of the clinical evolution and transmission of SARS-CoV-2 infection in cats by simulating natural routes of infection. Vet Res Commun. 2022;43:837-52. https://doi.org/10.1007/s11259-022-09908-5
Oxford Coronavirus Government Response Tracker. COVID-19 Tracker Global, América Latina y el Caribe, Colombia. Fecha de consulta: 20 de septiembre de 2021. Disponible en: https://graphics.reuters.com/world-coronavirus-tracker-and-maps/es/countries-and-territories/colombia/
World Health Organization. Diagnostic testing for SARS-CoV-2: Interim guidance. Fecha de consulta: 16 de marzo de 2021. Disponible en:. https://apps.who.int/iris/handle/10665/334254
Afzal A. Molecular diagnostic technologies for COVID-19: Limitations and challenges. J Adv Res. 2020;26:149-59. https://doi.org/10.1016/j.jare.2020.08.002
McHugh ML. Lessons in biostatistics interrater reliability: The kappa statistic. Biochem Med (Zagreb). 2012;22:276-82.
Giraldo-Ramírez S, Rendón-Marín S, Jaimes JA, Martínez-Gutiérrez M, Ruiz-Sáenz J. SARS-CoV-2 clinical outcome in domestic and wild cats: A systematic review. Animals. 2021;11:2056. https://doi.org/10.3390/ani11072056
Hulswit R, de Haan C, Bosch B. Coronavirus spike protein and tropism changes. In: Ziebuhr J, editor. Advances in virus research. Washington D. C.: Elsevier Inc.; 2016. p. 94-5. https://doi.org/10.1016/bs.aivir.2016.08.004
Muñoz M, Patiño LH, Ballesteros N, Paniz-Mondolfi A, Ramírez JD. Characterizing SARSCoV-2 genome diversity circulating in South American countries: Signatures of potentially emergent lineages? Int J Infect Dis. 2021;105:329-32. https://doi.org/10.1016/j.ijid.2021.02.073
Buitrago SP, Garzón-Ospina D. Genetic diversity of SARS-CoV-2 in South America: Demographic history and structuration signals. Arch Virol. 2021;166:3357-71. https://doi.org/10.1007/s00705-021-05258-w
Singer J. CoV-GLUE. nsp12 replacement S647I. Fecha de consulta: 23 de octubre de 2021. Disponible en: http://cov-glue.cvr.gla.ac.uk/#/project/replacement/NSP12:S:647:I
Hosie MJ, Epifano I, Herder V, Orton RJ, Stevenson A, Johnson N, et al. Detection of SARSCoV-2 in respiratory samples from cats in the UK associated with human-to-cat transmission. Vet Rec. 2021;188:e247. https://doi.org/10.1002/vetr.247
Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812-27.e19. https://doi.org/10.1016/j.cell.2020.06.043
Schlottau K, Rissmann M, Graaf A, Schön J, Sehl J, Wylezich C, et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe. 2020;1:e218-25. https://doi.org/10.1016/S2666-5247(20)30089-6
Some similar items:
- Jeison Monroy-Gómez, Orlando Torres-Fernández , Effects of the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) on the nervous system. What can we expect from SARS -CoV-2? , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Cristian Antony Ramos-Vera, Juan Camilo Motta, Letter to the editor "Prognostic factors in hospitalized patients diagnosed with SARS-CoV-2 infection, Bogotá, Colombia" , Biomedica: Vol. 41 No. 2 (2021)
- Juan Camilo Motta , Danny Julian Novoa, Carmen Cecilia Gómez, Julian Mauricio Moreno, Lina Vargas, Jairo Pérez, Henry Millán, Álvaro Ignacio Arango, Prognostic factors in hospitalized patients diagnosed with SARS-CoV-2 infection, Bogotá, Colombia , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Daniele Piovani , Georgios K. Nikolopoulos, Stefanos Bonovas, Pitfalls and perils of survival analysis under incorrect assumptions: the case of COVID-19 data , Biomedica: Vol. 41 No. Sp. 2 (2021): Octubre, Infecciones bacterianas y virales
- Gustavo Aroca, María Vélez-Verbel , Andrés Cadena , Lil Geraldine Avendaño , Sandra Hernández, Angélica Sierra, Omar Cabarcas, Santos Ángel Depine, COVID-19 in hemodialysis patients in Colombia: Report of seven cases , Biomedica: Vol. 40 No. Supl. 2 (2020): SARS-CoV-2 y COVID-19
- Alfredo G. Torres, Vaccines against SARS-CoV-2: Are they a reality for Latin America? , Biomedica: Vol. 40 No. 3 (2020)
- Gustavo Pradilla, Julio César Mantilla, Reynaldo Badillo, Human rabies encephalitis by a vampire bat bite in an urban area of Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Karol Liseth Rueda-Concha , Ana Payares-Mercado , Jesús Guerra-Castillo , Jesús Melendrez , Yasmit Arroyo-Munive, Lily Martínez-Abad , Suljey Cochero , Eduar Elías Bejarano , Luis Enrique Paternina, Silent circulation of Leishmania infantum and Trypanosoma cruzi among urban dogs from Sincelejo city, Caribbean region of Colombia , Biomedica: Vol. 42 No. 4 (2022)
- Richard C. Pacheco, Mauricio C. Horta, Jonas Moraes-Filho, Alexandre C. Ataliba, Adriano Pinter, Marcelo B. Labruna, Rickettsial infection in capybaras (Hydrochoerus hydrochaeris from São Paulo, Brazil: serological evidence for infection by Rickettsia bellii and Rickettsia parkeri , Biomedica: Vol. 27 No. 3 (2007)
- Jessika Valderrama, Ingrid García, Germán Figueroa, Edilberto Rico, Juliana Sanabria, Nicolás Rocha, Edgar Parra, Cecilia Saad, Andrés Páez, Outbreaks of human rabies transmitted by vampire bats in Alto Baudó and Bajo Baudó municipalities, department of Chocó, Colombia, 2004-2005 , Biomedica: Vol. 26 No. 3 (2006)
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
Funding data
-
Sistema General de Regalías de Colombia
Grant numbers BPIN 2020000100131
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























