Biochemical typing and evaluation of pathogenicity in vulvovaginal isolates of Candida albicans complex
Abstract
Introduction. Candida albicans, C. dubliniensis, and C. africana form the Candida albicans complex.
Objective. To identify the phenotypic and pathogenic characteristics of isolates of the C. albicans complex preserved in a collection.
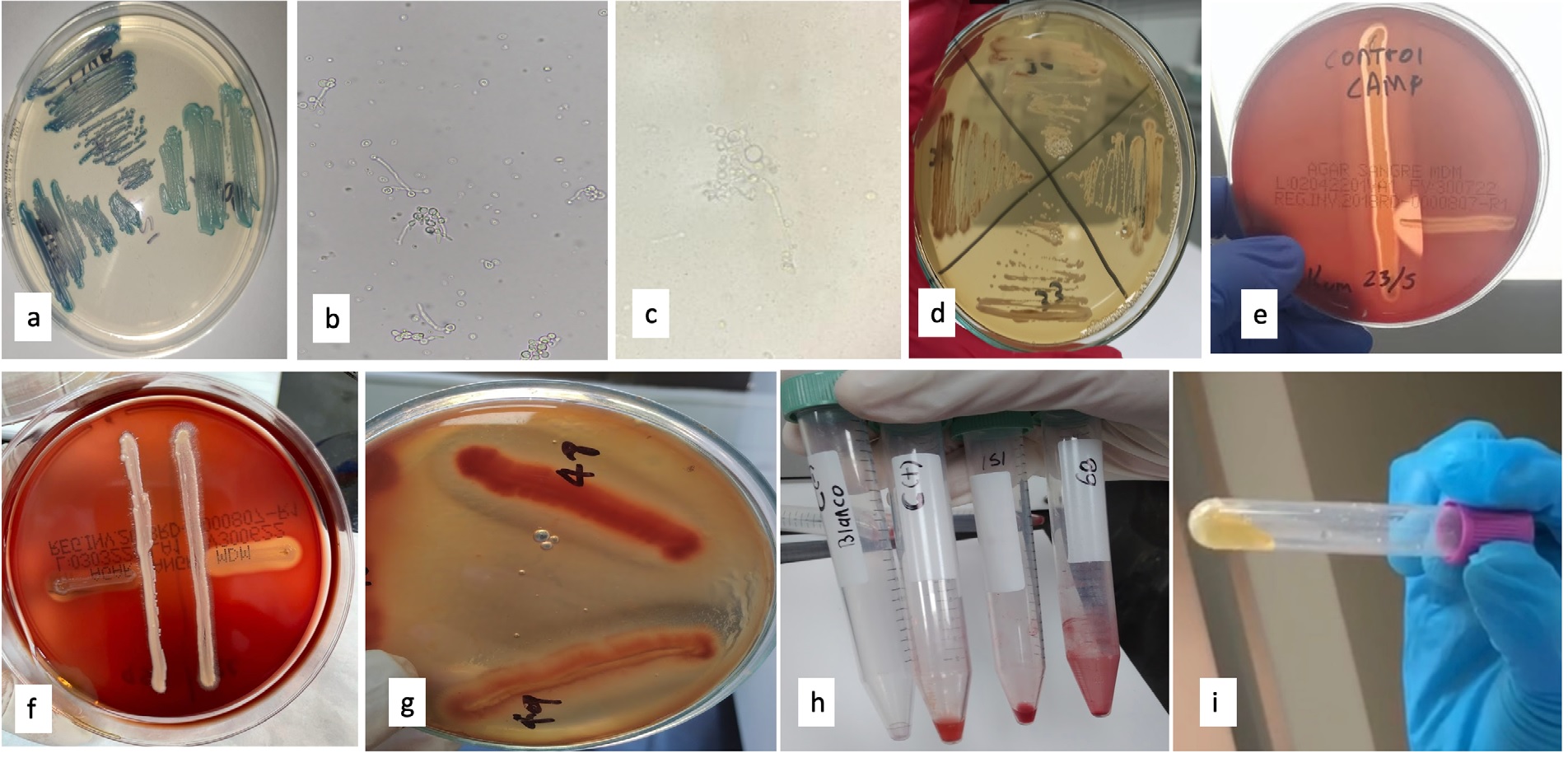
Materials and methods. Three hundred presumptive strains of the C. albicans complex were evaluated using CHROMagarTM Candida. Germ tube production was determined by
three methods, chlamydospores formation was assessed and colonies were characterized in artisanal agars (Rosmarinus officinalis and Nicotiana tabacum). MALDI-TOF was used as the gold standard identification test. To detect pathogenicity factors, we evaluated the hemolytic activity of each isolate and cocultured with Staphylococcus aureus, coagulase enzyme production, and biofilm formation.
Results. Out of the 300 isolates, 43.7% produced germ tube in the heart-brain infusion broth and 47% of the isolates produced chlamydospores. In the artisan media, 6% of the isolates produced brown colonies on rosemary agar and 5% did so on tobacco agar. None of the strains hemolyzed the blood agar alone or cocultured with S. aureus. However, 50% of the isolates hemolyzed the potato dextrose agar supplemented with blood. All strains were coagulase producers, and biofilm production was variable. For germ tube production, the human serum method showed the same positivity as the milk broth method. All isolates were identified as C. albicans by MALDI-TOF.
Conclusions. The use of proteomics, molecular tests or a combination of methods is required for species identification.
Downloads
References
Theill L, Dudiuk C, Morano S, Gamarra S, Nardin ME, Méndez E, et al. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev Argent Microbiol. 2016;48:43-9. https://doi.org/10.1016/j.ram.2015.10.003
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:1-20. https://doi.org/10.1038/nrdp.2018.26
Mucci MJ, Cuestas ML, Landanburu MF, Mujica MT. Prevalence of Candida albicans, Candida dubliniensis and Candida africana in pregnant women suffering from vulvovaginal candidiasis in Argentina. Rev Iberoam Micol. 2017;34:72-6. https://doi.org/10.1016/j.riam.2016.09.001
Vieille P. Identificación de cepas del complejo Candida albicans aisladas de muestras clínicas en la región de Valparaíso, Chile. Boletín Micológico. 2022;37:2-8. https://doi.org/10.22370/bolmicol.2022.37.1.3217n.
Rodríguez-Leguizamón G, Fiori A, López LF, Gómez BL, Parra-Giraldo CM, Gómez-López A, et al. Characterising atypical Candida albicans clinical isolates from six third-level hospitals in Bogotá, Colombia. BMC Microbiol. 2015;15:1-10. https://doi.org/10.1186/s12866-015-0535-0
Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. Candida dubliniensis sp. nov.: Phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:150721. https://doi.org/10.1099/13500872-141-7-1507
Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, et al. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231-44. https://doi.org/10.1101/gr.097501.109
Álvarez MI, Suárez BL, Caicedo LD. Isolation of Candida dubliniensis for the first time in Cali, Colombia, and its identification with phenotyping methods. Mycopathologia. 2009;167:19-24. https://doi.org/10.1007/s11046-008-9145-9
Tietz HJ, Hopp M, Schmalreck A, Sterry W, Czaika V. Candida africana sp. nov., a new human pathogen or a variant of Candida albicans? Mycoses. 2001;44:437-45. https://doi.org/10.1046/j.1439-0507.2001.00707.x
Hu Y, Yu A, Chen X, Wang G, Feng X. Molecular characterization of Candida africana in genital specimens in Shanghai, China. Biomed Res Int. 2015;185387. https://doi.org/10.1155/2015/185387
Yazdanparast SA, Khodavaisy S, Fakhim H, Shokohi T, Haghani I, Nabili M, et al. Molecular characterization of highly susceptible Candida africana from vulvovaginal candidiasis. Mycopathologia. 2015;180:317-23. https://doi.org/10.1007/s11046-015-9924-z
Nnadi NE, Ayanbimpe GM, Scordino F, Okolo M, Enweani IB, Criseo G, et al. Isolation and molecular characterization of Candida africana from Jos, Nigeria. Med Mycol. 2012;50:765-7. https://doi.org/10.3109/13693786.2012.662598
Romeo O, Criseo G. Candida africana and its closest relatives. Mycoses. 2011;54:475-86. https://doi.org/10.1111/j.1439-0507.2010.01939.x
Romeo O, Criseo G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn Microbiol Infect Dis. 2008;62:230-3. https://doi.org/10.1016/j.diagmicrobio.2008.05.014
Pakshir K, Bordbar M, Zomorodian K, Nouraei H, Khodadadi H. Evaluation of CAMP-like effect, biofilm formation, and discrimination of Candida africana from vaginal Candida albicans species. J Path. 2017;7126258. https://doi.org/10.1155/2017/7126258
Mba IE, Nweze EI. Mechanism of Candida pathogenesis: revisiting the vital drivers. Eur J Clin Microbiol Infect Dis. 2020;39:1797-819. https://doi.org/10.1007/s10096-020-03912-w
Pakshir K, Sheykhi S, Zomorodian K, Nouraei H, Zare Shahrabadi Z. Evaluation of biofilm formation in the homozygous and heterozygous strains of vaginal Candida albicans isolates. Curr Med Mycol. 2019;5:37-40. https://doi.org/10.18502/cmm.5.2.1160
Duarte A, Márquez A, Araújo C, Pérez C. Modalidades de la prueba del tubo germinal. Rev Soc Ven Microbiol. 2009;29:66-8.
De Loreto ES, Pozzatti P, Alves Scheid L, Santurio D, Morais Santurio J, Alves SH. Differentiation of Candida dubliniensis from Candida albicans on rosemary extract agar and oregano extract agar. J Clin Lab Anal. 2008;22:172-7. https://doi.org/10.1002/jcla.20237
Khan ZU, Ahmad S, Mokaddas E, Chandy R. Tobacco agar, a new medium for differentiating Candida dubliniensis from Candida albicans. J Clin Microbiol. 2004;42:4796-8. https://doi.org/10.1128/JCM.42.10.4796-4798.2004
Pineda G, Scollo K, Santiso G, Lehmann E, Arechavala A. Isolation of Candida dubliniensis in different clinical samples. Analysis of phenotypical methods to differentiate it from Candida albicans. Rev Argent Microbiol. 2008;40:211-7
Riceto EB de M, Menezes R de P, Penatti MPA, Pedroso R dos S. Enzymatic and hemolytic activity in different Candida species. Rev Iberoam Micol. 2015;32:79-82. https://doi.org/10.1016/j.riam.2013.11.003
Döğen A, Gümral R, Ilkit M. Haemolytic and co-haemolytic (CAMP-like) activity in dermatophytes. Mycoses. 2015;58:40-7. https://doi.org/10.1111/myc.12269
Rodrigues AG, Pina-Vaz C, Costa-de-Oliveira S, Tavares C. Expression of plasma coagulase among pathogenic Candida species. J Clin Microbiol. 2003;41:5792-3. https://doi.org/10.1128/JCM.41.12.5792-5793.2003
Teke L, Barış A, Bayraktar B. Comparative evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for non-albicans Candida and uncommon yeast isolates. J Microbiol Methods. 2021;185:106232. https://doi.org/10.1016/j.mimet.2021.106232
Guarro J. Taxonomía y biología de los hongos causantes de infección en humanos. Enferm Infecc Microbiol Clin. 2012;30:33-9. https://doi.org/10.1016/j.eimc.2011.09.006
Ballesté R, Arteta Z, Fernández N, Cristina M, Mousqués N, Xavier B, et al. Evaluación del medio cromógeno CHROMagarTM Candida para la identificación de levaduras de interés médico. Rev Med Urug (Montev). 2005;21:186-93.
Alfonso C, López M, Arechavala A, del Carmen Perrone M, Guelfand L, Bianchi M. Identificación presuntiva de Candida spp. y de otras levaduras de importancia clínica: utilidad de brilliance Candida agar. Rev Iberoam Micol. 2010;27:90-3. https://doi.org/10.1016/j.riam.2010.01.008
Odds FC, Davidson A. “Room temperature” use of CHROMagar Candida. Diagn Microbiol Infect Dis. 2000;38:147-50. https://doi.org/10.1016/s0732-8893(00)00197-8
Madhavan P, Jamal F, Chong PP, Ng KP. Identification of local clinical Candida isolates using CHROMagarTM Candida as a primary identification method for various Candida species. Trop Biomed. 2011;28:269-74.
Vecchione A, Florio W, Celandroni F, Barnini S, Lupetti A, Ghelardi E. Comparative evaluation of six chromogenic media for presumptive yeast identification. J Clin Pathol. 2017;70:1074-8. https://doi.org/10.1136/jclinpath-2017-204396
Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329-34. https://doi.org/10.1128/JCM.36.2.329-334.1998
Yazdanpanah A, Khaithir TMN. Issues in identifying germ tube positive yeasts by onventional methods. J Clin Lab Anal. 2014;28:1-9. https://doi.org/10.1002/jcla.21635
Linares S, Solís F. Identificación de levaduras. En: Pemán J, Martín-Mazuelo E, Rubio MC. Guía práctica de identificación y diagnóstico en micología clínica. Segunda edición. Madrid: Bilbao; 2007. p.1-20.
Feo M, De Pacheco A. Candida albicans: la leche en la producción de clamidosporas. Am Microbiol. 1976;18:23-4.
Casal M, Linares MJ. Comparations of six media for production of chlamidospore by Candida albicans. Mycopathologia. 1981;76:125-8.
Reyes KJ, Araiza J, Bonifaz A. Formación de clamidoconidios de Candida albicans y C. dubliniensis en diferentes medios líquidos y condiciones de incubación. Scientia Fungorum. 2007;25:27-31.
Silveira-Gomes F, Sarmento DN, Espírito-Santo EPTD, Souza NDO, Pinto TM, Marquesda-Silva SH. Differentiation between Candida albicans and Candida dubliniensis usinghypertonic Sabouraud broth and tobacco agar. Rev Soc Bras Med Trop. 2011;44:457-60. https://doi.org/10.1590/s0037-86822011000400011
Sardi JC, Duque C, Höfling JF, Gonçalves RB. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Sabouraudia. 2012;50:467-75. https://doi.org/10.3109/13693786.2011.633233
Luo G, Samaranayake LP, Yau JY. Candida species exhibit differential in vitro hemolytic activities. J Clin Microbiol. 2001;39:2971-4. https://doi.org/10.1128/JCM.39.8.2971-2974.2001
Nouraei H, Pakshir K, ZareShahrabadi Z, Zomorodian K. High detection of virulence factors by Candida species isolated from bloodstream of patients with candidemia. Microb Pathog. 2020;149:104574 https://doi.org/10.1016/j.micpath.2020.104574
Wan L, Luo G, Lu H, Xuan D, Cao H, Zhang J. Changes in the hemolytic activity of Candida species by common electrolytes. BMC Microbiol. 2015;15:1-7. https://doi.org/10.1186/s12866-015-0504-7
El-Baz AM, Mosbah RA, Goda RM, Mansour B, Sultana T, Dahms TE, ElGaniny AM. Back to nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics (Basel). 2021;10:81-98. https://doi.org/10.3390/antibiotics10010081
Noori M, Dakhili M, Sepahvand A, Davari N. Evaluation of esterase and hemolysin activities of different Candida species isolated from vulvovaginitis cases in Lorestan Province, Iran. Curr Med Mycol. 2017;3:1-5. https://doi.org/10.29252/cmm.3.4.1
Maheronnaghsh M, Fatahinia M, Dehghan P, Mahmoudabadi AZ, Kheirkhah M. Comparison of virulence factors of different candida species isolated from the oral cavity of cancer patients and normal individuals. Jundishapur J Microbiol. 2019;12: e91556. https://doi.org/10.5812/jjm.91556
Manns JM, Mosser DM, Buckley HR. Production of a hemolytic factor by Candida albicans. Infect Immun. 1994;62:5154-6 https://doi.org/10.1128/iai.62.11.51545156.1994
Mohammadi F, Hemmat N, Bajalan Z, Javadi A. Analysis of biofilm-related genes and antifungal susceptibility pattern of vaginal Candida albicans and Non-Candida albicans species. Biomed Res Int. 2021:5598907. https://doi.org/10.1155/2021/5598907
Kokare CR, Chakraborty S, Khopade AN, Mahadik KR. Biofilm: Importance and applications. Indian J Biotechnol. 2009;8:159-68.
Ganguly S, Mitchell AP. Mucosal biofilms of Candida albicans. Curr Opin Microbiol. 2011;14:380-5 https://doi.org/10.1016/j.mib.2011.06.001
Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, et al. Biofilmforming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis. 2017;23:328-31 https://doi.org/10.3201/eid2302.161320
Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475-9. https://doi.org/10.1128/AAC.45.9.2475-2479.2001
Rodríguez-Cerdeira C, Gregorio MC, Molares-Vila A, López-Barcenas A, Fabbrocini G, Bardhi B, et al. Biofilms and vulvovaginal candidiasis. Colloids Surf B Biointerfaces. 2019;174:110-25. https://doi.org/10.1016/j.colsurfb.2018.11.011
Noumi E, Snoussi M, Noumi I, Saghrouni F, Aouni M, Valentín E. Phenotypic characterization and adhesive properties of vaginal Candida spp. strains provided by the CHU Farhat Hached (Sousse, Tunisia). Rev Iberoam Micol. 2012;32:170-9. https://doi.org/10.1016/j.riam.2014.06.006
Seifi Z, Mahmoudabadi AZ, Zarrin M. Extracellular enzymes and susceptibility to fluconazole in Candida strains isolated from patients with vaginitis and healthy individuals. Jundishapur J Microbiol. 2015;8:e20162. https://doi.org/10.5812/jjm.20162
Yigit N, Aktas E, Dagistan S, Ayyildiz A. Investigating biofilm production, coagulase and hemolytic activity in Candida species isolated from denture stomatitis patients. Eurasian J Med. 2011;43:27-32. https://doi.org/10.5152/eajm.2011.06
Hazirolan G, Altun HU, Gumral R, Gursoy NC, Otlu B, Sancak B. Prevalence of Candida fricana and Candida dubliniensis, in vulvovaginal candidiasis: first Turkish Candida africana isolates from vulvovaginal candidiasis. J Mycol Med.2017;27:376-81. https://doi.org/10.1016/j.mycmed.2017.04.106
Some similar items:
- Husein Husein-El Ahmed, Guillermo Arturo Cañadas-De la Fuente, Rafael Fernández-Castillo, Emilio González-Jiménez, Jesús Cantero-Hinojosa, Marita Lardón-Fernández, Generalized cutaneous candidiasis in newborn at term , Biomedica: Vol. 32 No. 2 (2012)
- Javier Araiza , Valentín Sánchez-Pedraza, Ana Karen Carrillo , Denise Fernández-Samar, Jazmín Tejeda, Alexandro Bonifaz, Mixed oral candidiasis in type 2 diabetic patients: Identification and spectrum of sensitivity , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Ana Victoria Suescún, Juan Rodrigo Cubillos, María Mercedes Zambrano, Genes involved in fimbrial biogenesis affect biofilm formation in Klebsiella pneumoniae , Biomedica: Vol. 26 No. 4 (2006)
- Catalina de Bedout, Julio Ayabaca, Ricardo Vega, Matilde Méndez, Axel R. Santiago, María Lucrecia Pabón, Angela Tabares, Myrtha Arango, Angela Restrepo, Vance Newell, Evaluation of Candida species' susceptibility to fluconazole with the disk diffusion method. , Biomedica: Vol. 23 No. 1 (2003)
- Liliana Torcoroma García, Liany Johanna Luna, Tania Katherine Velasco, Beatriz Elena Guerra, new multiplex PCR for species-specific diagnosis of human candidiasis , Biomedica: Vol. 37 No. 2 (2017)
- Ayerim García, Carlos Martínez, Rosa Isela Juárez, René Téllez, Marco Antonio Paredes, María del Rocío Herrera, Silvia Giono, Methicillin resistance and biofilm production in clinical isolates of Staphylococcus aureus and coagulase-negative Staphylococcus in México , Biomedica: Vol. 39 No. 3 (2019)
- Ana Elisa Rojas, Jorge Enrique Pérez, Johan Sebastián Hernández, Yuliana Zapata, Quantitative analysis of the expression of fluconazole-resistant genes in strains of Candida albicans isolated from elderly people at their admission in an intensive care unit in Manizales, Colombia , Biomedica: Vol. 40 No. 1 (2020)
- Jorge Alberto Cortés, José Franklin Ruiz, Lizeth Natalia Melgarejo-Moreno, Elkin V. Lemos, Candidemia in Colombia , Biomedica: Vol. 40 No. 1 (2020)
- Xiomara Moreno , Melanie Ventura, María Mercedes Panizo, María Fátima Garcés, Assessment of biofilms formation of bacterial and fungal isolates using qualitative Congo red agar and semiquantitative crystal violet microtiter methods , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
Copyright (c) 2023 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
Funding data
-
Ministerio de Ciencia y Tecnología
Grant numbers 891 de 2020
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























