Polyclonal antibodies against recombinant dengue virus NS3 protein
Abstract
Introduction: Dengue is a disease caused by one of four serotypes of the dengue virus (DENV) and is endemic in approximately 130 countries. The incidence of dengue has increased dramatically in recent decades, as well as the frequency and magnitude of outbreaks. Despite all efforts, there are no prophylactic or therapeutic treatments for the disease. Accordingly, research on the processes governing the DENV infection cycle is essential to develop vaccines or antiviral therapies. One of the
most attractive DENV molecules to investigate is nonstructural protein 3 (NS3), which is essential for viral replication and a major immune target for infection.
Objective: To produce antibodies to support future studies on NS3 and its cellular interactions with other proteins.
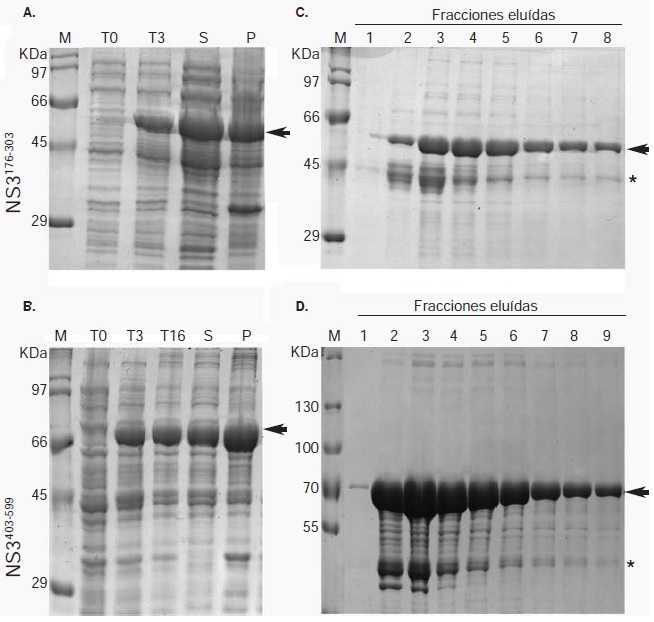
Materials and methods: Two recombinant proteins of the helicase domain of DENV NS3 serotype 2 were expressed, and used to immunize mice and produce polyclonal antibodies.
Results: The antibodies produced were useful in Western blot and immunofluorescence tests. We report an NS3 antibody that immunoprecipitates the viral protein and detects it in Western blot with no need to over-express it or use cell extracts with metabolic radiolabeling.
Conclusion: The recombinant proteins expressed and the antibodies produced constitute valuable tools for studying DENV infectious processes involving NS3 and for evaluating tests designed to interfere with its functions.
Downloads
References
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504-7. http://dx.doi.org/10.1038/nature12060
Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649-88. http://dx.doi.org/10.1146/
annurev.mi.44.100190.003245
Velandia ML, Castellanos JE. Virus del dengue: estructura y ciclo viral. Infectio. 2011;15:33-43. http://dx.doi.org/10.1016/S0123-9392(11)70074-1
Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991;65:2467-75.
Li H, Clum S, You S, Ebner KE, Padmanabhan R. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J Virol. 1999;73:3108-16.
Bartelma G, Padmanabhan R. Expression, purification, and characterization of the RNA 5′-triphosphatase activity of dengue virus type 2 nonstructural protein 3. Virology. 2002;299:122-32. http://dx.doi.org/10.1006/viro.2002.1504
Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci USA. 2010;107:16922-27. http://dx.doi.org/10.1073/pnas.1010867107
Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue infection. J Virol. 2012;87:2693-706. http://dx.doi.org/10.1128/JVI.02675-12
Chien LJ, Liao TL, Shu PY, Huang JH, Gubler DJ, Chang GJ. Development of real-time reverse transcriptase PCR assays to detect and serotype dengue viruses. J Clin Microbiol. 2006;44:1295-304. http://dx.doi.org/10.1128/JCM.44.4.1295-1304.2006
Costa SM, Yorio AP, Gonҫalves AJ, Vidale MM, Costa EC, Mohana-Borges R, et al. Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines. PloSOne. 2011;6:e25685. http://dx.doi.org/10.1371/journal.pone.0025685
Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: Application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20-8. http://dx.doi.org/10.1083/jcb.99.1.20
Zou J, Wang QY, Xie X, Lu S, Yau YH, Yuan Z, et al. Mapping the interactions between the NS4B and NS3 proteins of dengue virus. J Virol. 2015;89:3471-83. http://dx.doi.org/10.1128/JVI.03454-14
Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and
NS4A. Virology. 1998;245:203-15. http://dx.doi.org/10.1006/viro.1998.9156
Kapoor M, Zhang L, Ramachandra M, Kusukawa J, Ebner KE, Padmanabhan R. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J Biol Chem. 1995;270:19100-6. http://dx.doi.org/10.1074/jbc.270.32.19100
Chua JJ, Ng MM, Chow VT. The non-structural 3 (NS3) protein of dengue virus type 2 interacts with human nuclear receptor binding protein and is associated with alterations in membrane structure. Virus Res. 2004;102:151-63. http://dx.doi.org/10.1016/j.virusres.2004.01.025
Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn R J, et al. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci USA. 2010;107:17345-50. http://dx.doi.org/10.1073/pnas.1010811107
Arias CF, Preugschat F, Strauss JH. Dengue 2 virus NS2B and NS3 form a stable complex that can cleave NS3 within the helicase domain. Virology. 1993;193:888-99. http://dx.doi.org/10.1006/viro.1993.1198
Teo KF, Wright PJ. Internal proteolysis of the NS3 protein specified by dengue virus 2. J Gen Virol. 1997;78:337-41. http://dx.doi.org/10.1099/0022-1317-78-2-337
Some similar items:
- Marcel Marín, Yudy Alexandra Aguilar, José Robinson Ramírez, Omar Triana, Carlos Enrique Muskus, Molecular and immunological analyses suggest the absence of hydrophilic surface proteins in Leishmania (Viannia) panamensis , Biomedica: Vol. 28 No. 3 (2008)
- Carmenza Macía, Sandra Vargas, Ana María Mora, Ashly Melissa Sarmiento, Robinson Pacheco, Fernando Rosso, Seroprevalence of human T-lymphotropic virus in blood bank donors at Fundación Valle del Lili, Cali, Colombia, 2008-2014 , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Claudia M.E. Romero, Humberto Llinás, Andrew K.I. Falconar, Evaluation of a rapid water-surface sweeping method to curately estimate numbers of Aedes aegypti (Diptera: Culicidae) late larval stages in large water-storage containers: comparison with pupal estimates , Biomedica: Vol. 30 No. 2 (2010)
- Raquel E. Ocazionez-Jiménez, Ayda Susana Ortiz-Báez, Sergio Yebrail Gómez-Rangel, Daniel R. Miranda-Esquivel, Dengue virus serotype 1 (DENV-1) from Colombia: its contribution to dengue occurrence in Santander , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- Berlin Londoño-Rentería, Jenny C. Cárdenas, Jeniffer E. Giovanni, Lucio Cárdenas, Paloma Villamizar, Jenniffer Rolón, Daniel M. Chisenhall, Rebecca C. Christofferson, Daysi J. Carvajal, Omar G. Pérez, Dawn M. Wesson, Christopher N. Mores, Aedes aegypti anti-salivary gland antibody concentration and dengue virus exposure history in healthy individuals living in an endemic area in Colombia , Biomedica: Vol. 35 No. 4 (2015)
- Mauricio Hernández, Diana Arboleda, Stephania Arce, Allan Benavides, Paola Andrea Tejada, Sindy Vanessa Ramírez, Ángela Cubides, Methodology to develop endemic channels and notification trends for dengue in Valle del Cauca, Colombia, 2009-2013 , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Juliana Pérez-Pérez, William H. Sanabria, Carolina Restrepo, Raúl Rojo, Enrique Henao, Omar Triana, Ana María Mejía, Sandra M. Castaño, Guillermo L. Rúa-Uribe, Virological surveillance of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus as support for decision making for dengue control in Medellín , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Andrés Gómez-Palacio, Juan Suaza-Vasco, Sandra Castaño, Omar Triana, Sandra Uribe, Aedes albopictus (Skuse, 1894) infected with the American-Asian genotype of dengue type 2 virus in Medellín suggests its possible role as vector of dengue fever in Colombia , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Myriam Lucía Velandia-Romero, Víctor Alberto Olano, Carolina Coronel-Ruiz, Laura Cabezas, María Angélica Calderón-Peláez, Jaime Eduardo Castellanos, María Inés Matiz, Dengue virus detection in Aedes aegypti larvae and pupae collected in rural areas of Anapoima, Cundinamarca, Colombia , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Yda Méndez, César Pacheco, Flor Herrera, Inhibition of defensin A and cecropin A responses to dengue virus 1 infection in Aedes aegypti , Biomedica: Vol. 41 No. 1 (2021)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























