In vitro culture of L3 larvae of nematodes obtained from the African giant snail Lissachatina fulica (Mollusca: Gastropoda) in Santa Fe de Antioquia
Abstract
Introduction: Over 170 municipalities in Colombia have been invaded by Lissachatina fulica, an African snail that can carry larvae of nematodes of interest in human and veterinary health. Nematodes enter the host snail as larvae L1 and then change to L2 and L3, the infectious form for vertebrates.
Objective: To standardize culture in vitro of L3 carried by L. fulica from Santa Fe de Antioquia.
Materials and methods: Between July and November, 2014, 10 snails were collected, killed, and conserved with HCl 0.7%. Larvae were recovered using the Baermann technique and cultured for 36 days in Schneider, DMEM and RPMI media, with and without SFB 20% and distilled water with SFB 20%. Replacements were made every 36 hours; larvae were measured with an ocular micrometer on a microscope. Summary statistics were estimated; box and whisker plots were made; the t Student test was performed in SPSS 18™. A p-value below 0.05 was assumed as significant.
Results: Fifty per cent of the larvae survived. The highest survival and growth was 85% in supplemented DMEM. The final average length of larvae in supplemented media exceeded the initial one. There were significant differences between the average length of larvae cultured in supplemented media and the initial length. The initial width of larvae did not change.
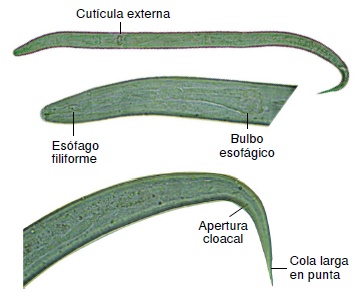
Conclusions: The best medium for the culture of L3 larvae was supplemented DMEM. The length provided more information than the width for the larval growth evaluation. The larvae studied did not correspond to Angiostrongylus cantonensis, A. costaricensis or Aelurostrongylus abstrusus.
Downloads
References
Wang Q, Wu D, Wei J, Owen R, Lun Z. Human Angiostrongylus cantonensis: An update. Eur J Clin Microbiol Infect Dis. 2012;31:389-95. https://doi.org/10. 1007/s10096-011-1328-5
De Oliveira A, Torres E, Maldonado J, de Barros J, Fernandez M, Thiengo S. Achatina fulica como hospedeiro intermediário de nematódeos de interesse médico-veterinário em Goiás, Brasil. Rev Pat Trop. 2010;39:199-210.
Fontanilla I. Achatina (Lissachatina) fulica Bowdich: Its molecular phylogeny, genetic variation in global populations, and its possible role in the spread of the rat lungworm Angiostrongylus cantonensis (CHEN) (tesis). Nottingham: University of Nottingham; 2010.
Rodríguez G. Hematoquecia letal por angiostrongilosis abdominal. Biomédica. 2000;20:120-30. https://doi.org/10.7705/biomedica.v20i2.1055
De La Ossa A, Carlos J, Lasso A. Registro del caracol africano gigante Achatina fulica (Bowdich 1822) (Mollusca: Gastropoda-Achatinidae) en Sincelejo, costa Caribe de Colombia. Biota Colombiana. 2012;13:247-52.
Linares E, Avendaño J, Martínez A, Rojas A. El caracol gigante africano, un visitante indeseado. Miniguía de campo. Bogotá: Universidad Nacional de Colombia; 2013.
Vogler RE, Beltramino AA, Sede MM, Gregoric DE, Núñez V, Rumi A. The giant African snail, Achatina fulica (Gastropoda: Achatinidae): Using bioclimatic models to identify South American areas susceptible to invasion. Amer Malac Bull. 2013;31:39-50. https://doi.org/10.4003/006.031.0115
Sistema Nacional Argentino de Vigilancia y Monitoreo de Plagas. Sistema de Prevención, Monitoreo y Control del Caracol Gigante Africano (Achatina fulica). Fecha de consulta: 9 de enero de 2015. Disponible en: http://www.sinavimo.gov.ar/pagina/sistema-de-prevencion-monitoreoy-control-del-caracol-gigante-africano-achatina-fulica
Fontanilla IK, Sta María IM, García J, Ghate H, Naggs F, Wade C. Restricted genetic variation in populations of Achatina (Lissachatina) fulica outside of East Africa and the Indian Ocean Islands points to the Indian Ocean Islands as the earliest known common source. PLoS One. 2014;9:e105151. https://doi.org/10.1371/journal.pone.0105151
Thiengo S, Maldonado A, Mota E, Torres E, Caldeira R, Carvalho O, et al. The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop. 2010;115:194-9. https://doi.org/10.1016/j.actatropica.2010.01.005
Contreras A, Núñez F, Pérez O, Lastre M, Magraner M, Bu-Coifiu R, et al. Peculiaridades de la meningoencefalitis por Angiostrongylus cantonensis en América. Rev Neurol. 2007;45:755-63.
Maldonado A, Simoes R, Oliveira A, Motta E, Fernandez M, Pereira Z, et al. Firts report of Angiostrongylus cantonensis (Nematoda: Metastrongyloidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz. 2010;105: 938-41. https://doi.org/10.1590/S0074-02762010000700019
Coaglio Silva LA. Susceptibilidade e comportamento de Achatina fulica infectada com Angiostrongylus vasorum (tesis). Belo Horizonte: Universidade Federal de Minas Gerais; 2013.
Thiengo S, Fernandez M, Torres E, Coelho P, Lanfredi R. First record of a nematode Metastrongyloidea (Aelurostrongylus abstrusus larvae) in Achatina (Lissachatina) fulica (Mollusca, Achatinidae) in Brazil. J Inverteb Pathol. 2008;98:34-9. https://doi.org/10.1016/j.jip.2007.10.010
Corantioquia. Un peligroso caracol gigante africano nos invade. Santa Fe de Antioquia. Fecha de consulta: 9 de noviembre de 2014. Dosponible en: http://nuevoportal.corantioquia.gov.co/Lists/Noticias%20Izquierda/Detalle.aspx?ID=3&ContentTypeId=0x010400852056182E12584AA4A86AF5CD531A56
Corantioquia. Informe final del Convenio Nº 792 de 2012, suscrito entre la Universidad de Antioquia y la Corporación Autónoma Regional del Centro de Antioquia. Medellín: Corantioquia; 2014. p. 78.
Smyth JD. In vitro cultivation of parasitic helminths. Boca Ratón: CRC Press; 1990. p. 1-144.
Yasuraoka K, Hata H. In vitro cultivation of parasitic helminths. Prog Med Parasitol Jpn. Tokyo: Meguro Parasitological Museum; 2003. p. 211-26.
Lin R, He J, Chung L, Lee J, Wang J, Yen C. Angiostrongylus cantonensis (Nematode: Metastrongiloidea): In vitro cultivation of infective third-stage larvae to fourth-stage larvae. PLoS One. 2013;8:e72084. https://doi.org/10.1371/journal.pone.0072084
Ahmed N. Cultivation of parasites. Trop Parasitol. 2014;4:80-9. https://doi.org/10.4103/2229-5070.138534
Ash LR. Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J Parasitol. 1970;56:249-53. https://doi.org/10.2307/3277651
Thiengo SC, Vicente JJ, Pinto RM. Redescription of Angiostrongylus (Paranstrongylus) costaricensis Morera & Céspedes (Nematoda: Metastrongyloidea) from Brazilian strain. Rev Bras Zool. 1997;14:839-44. https://doi.org/10.1590/S0101-81751997000400008
Hata H. In vitro cultivation of the third and fourth stage larvae of Angiostrongylus cantonensis. J Vet Med Sci. 1993;55:345-7. https://doi.org/10.1292/jvms.55.345
Some similar items:
- John A. Patiño, Mario J. Olivera, Gastro-allergic anisakiasis: The first case reported in Colombia and a literature review , Biomedica: Vol. 39 No. 2 (2019)
- Constanza Pardo, Ricardo Cendales, Survival analysis of cervical cancer patients , Biomedica: Vol. 29 No. 3 (2009)
- Raúl Murillo, Ricardo Cendales, Carolina Wiesner, Marion Piñeros, Sandra Tovar, Effectiveness of cytology-based cervical cancer screening in the Colombian health system , Biomedica: Vol. 29 No. 3 (2009)
- Sandra Lorena Girón, Julio César Mateus, Fabián Méndez, Impact of an open waste disposal site on the occurrence of respiratory symptoms and on health care costs of children , Biomedica: Vol. 29 No. 3 (2009)
- José Joaquín Carvajal, Ligia Inés Moncada, Mauricio Humberto Rodríguez, Ligia del Pilar Pérez, Víctor Alberto Olano, Characterization of Aedes albopictus (Skuse, 1894) (Diptera:Culicidae) larval habitats near the Amazon River in Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- Gustavo Pradilla, Julio César Mantilla, Reynaldo Badillo, Human rabies encephalitis by a vampire bat bite in an urban area of Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Mauricio Beltrán, María Cristina Navas, María Patricia Arbeláez, Jorge Donado, Sergio Jaramillo, Fernando De la Hoz, Cecilia Estrada, Lucía del Pilar Cortés, Amalia de Maldonado, Gloria Rey, Seroprevalence of hepatitis B virus and human immunodeficiency virus infection in a population of multiply-transfused patients in Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Rosa Magdalena Uscátegui, Adriana M. Correa, Jaime Carmona-Fonseca, Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria , Biomedica: Vol. 29 No. 2 (2009)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























