In vitro susceptibility of Cuban Aspergillus spp. strains of clinical and environmental origin
Abstract
Introduction: The behavior of antifungal susceptibility of Aspergillus spp. in Cuba remains unknown. The antifungals recommended to treat aspergillosis are amphotericin B, itraconazole, voriconazole and echinocandins. The influence of the environment may set off the emergence of drug-resistance in these microorganisms.
Objective: To evaluate in vitro susceptibility of Aspergillus spp. strains to amphotericin B, itraconazole and voriconazol, and the relationship between susceptibility patterns and their origin.
Materials and methods: Minimum inhibitory concentrations of amphotericin B, itraconazole and voriconazole were determined for 60 Aspergillus spp. strains of clinical and environmental origin using the M38-A2 method of the Clinical and Laboratory Standards Institute.
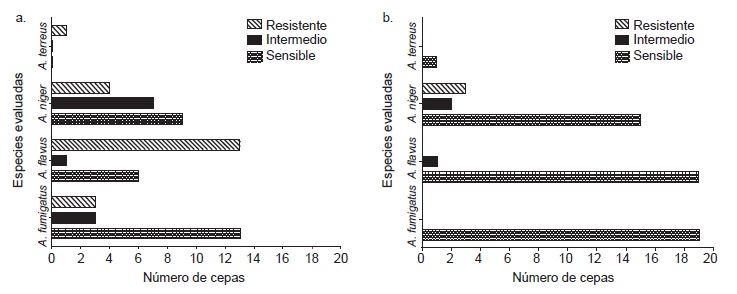
Results: We found 21 amphotericin B resistant strains (mainly from clinical samples and hospital environments), as well as three itraconazole resistant strains (from non-hospital outdoor and indoor environments). No voriconazole resistance was found. No relationship was found between strain origin and susceptibility.
Conclusions: Results suggest the possible existence of environmental factors or interactions with resistant genotypes which may give rise to resistant phenotypes in our country. This is the first report of in vitro Aspergillus spp. resistant strains in Cuba. These studies should be broadened and include molecular and phylogenetic analyses.
Downloads
References
Bennett JW. Aspergillus: A primer for the novice. Med Mycol. 2009;47:5-12. https://doi.org/10.1080/13693780802712515
Stevens DA. Clinical aspergillosis for basic scientists. Med Mycol. 2009;47:1-4. https://doi.org/10.1080/13693780 802322232
Patterson TF, Thompson III GR, Denning DW, Fishman JA, Hadley S, Herbercht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016:63:1-60. https://doi.org/10.1093/cid/ciw326
Pham CD, Reiss E, Hagen F, Meis JF, Lockhart SR. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011-2013. Emerg Infect Dis. 2014;20:1498-503. https://doi.org/10.3201/eid2009.140142
Badiee P, Alborzi A, Moeini M, Haddadi P, Farshad S, Japoni A, et al. Antifungal susceptibility of the Aspergillus species by Etest and CLSI reference methods. Arch Iran Med. 2012;15:429-32.
Lass-Flörl C, Perkhofer S. In vitro susceptibility-testing in Aspergillus species. Mycoses. 2008;51:437-46. https://doi.org/10.1111/j.1439-0507.2008.01510.x.
Barnet HL, Hunter BB. Illustrated genera of imperfect fungi. Portland: MacMillan Publisher Co.; 1997. p. 218.
Klich MA, Pitt JI. A laboratory guide to the common Aspergillus species and their 19 teleomorphs. Canberra: CSIRO - Division of Food Processing; 2002. p. 116.
De Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of Clinical Fungi. Segunda edición. Centraalbureau voor Schimmelcultures, Utrecht: Universitat Rovira i Virgili, Reus; 2000. p. 442-519.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Document M38-A2; Approved Standard. Second edition. Wayne: Clinical and Laboratory Standards Institute; 2008. p. 52.
Guinea J, Peláez T, Alcalá L, Bouza E. Correlation between the ETest and the CLSI M38-A microdilution method to determine the activity of amphotericin B, voriconazole, and itraconazole against clinical isolates of Aspergillus fumigatus. Diagn Micr Infec Dis. 2007;57:273-6. https://doi.org/10.1016/j.diagmicrobio.2006.09.003
Chakrabarti A. Drug resistance in fungi: An emerging problem. Regional Health Forum. 2011;15:97-103.
Ellis D. Amphotericin B: Spectrum and resistance. J Antimicrob Chemother. 2002;49:7-10. https://doi.org/10. 1093/jac/49.suppl_1.7
Knechtel SA, Klepser ME. Amphotericin B resistance: Epidemiology, mechanisms, and clinical relevance. J Invasive Fungal Infect. 2007;1:93-8.
Guarro J, Xavier MO, Severo LC. Differences and similarities amongst pathogenic Aspergillus species. En: Pasqualotto AC, editor. Aspergillosis: From diagnosis to prevention. Dordrecht: Springer; 2010. p. 7-32.
Lass-Flörl C, Izquierdo AA, Cuenca-Estrella M, Perkhofer S, Tudela JLR. In vitro activities of various antifungal drugs against Aspergillus terreus: Global assessment using the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother. 2009; 53:794-5. https://doi.org/10.1128/AAC.00335-08
Krishnan S, Manavathu EK, Chandrasekar PH. Aspergillus flavus: An emerging non- fumigatus Aspergillus species of significance. Mycoses. 2009;52:206-22. https://doi.org/10. 1111/j.1439-0507.2008.01642.x
Seo K, Akiyoshi H, Ohnishi Y. Alteration of cell wall com-position leads to amphotericin B resistance in Aspergillus flavus. Microbiol Immunol. 1999;43:1017-25. https://doi.org/10. 1111/j.1348-0421.1999.tb01231.x
Howard SJ, Harrison E, Bowyer P, Varga J, Denning DW. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob Agents Chemother. 2011;55:4802-9. https://doi.org/10.1128/AAC.00304-11
Kaya AD, Kiraz N. In vitro susceptibilities of Aspergillus spp. causing otomycosis to amphotericin B, voriconazole and itraconazole. Mycoses. 2007;50:447-50. https://doi.org/10.1111/j.1439-0507.2007.01409.x
Gheith S, Saghrouni F, Bannour W, Ben Youssef Y, Khelif A, Normand AC, et al. In vitro susceptibility to amphotericin B, itraconazole, voriconazole, posaconazole and caspofungin of Aspergillus spp. isolated from patients with haematological malignancies in Tunisia. Springerplus. 2014;3:1-8. https://doi.org/10.1186/2193-1801-3-19
Almaguer M, Rojas-Flores TI. Aeromicota viable de la atmósfera de La Habana, Cuba. Nova Acta Cient Compostel Biol. 2013;20:35-45.
Rojas TI, Martínez E, Aira MJ, Almaguer M. Aeromicota de ambientes internos: comparación de métodos de muestreo. Boletín Micológico. 2008;23:67-73.
Bernal-Martínez L, Alastruey-Izquierdo A, Cuenca-Estrella M. Diagnostics and susceptibility testing in Aspergillus. Future Microbiol. 2016;11:315-28. https://doi.org/10.2217/fmb.15.140
Vadlapudi V. Antifungal resistance of few Aspergillus species. Pharmacophore. 2011;2:163-7.
van der Linden JW, Warris A, Verweij PE. Aspergillus species intrinsically resistant to antifungal agents. Med Mycol. 2011;49:82-9. https://doi.org/10.3109/13693786.2010.499916
Tokarzewski S, Ziółkowska G, Nowakiewicz A. Suscep-tibility testing of Aspergillus niger strains isolated from poultry to antifungal drugs – a comparative study of the disk diffusion, broth microdilution (M 38-A) and Etest® methods. Pol J Vet Sci. 2012;15:125-33. https://doi.org/10.2478/v10181-011-0123-7
Pfaller M, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ, et al. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J Clin Microbiol. 2008;46:2568-72. https://doi.org/10.1128/JCM.00535-08
Pemán J, Salavert M, Cantón E, Jarque I, Romá E, Zaragoza R, et al. Voriconazole in the management of nosocomial invasive fungal infections. Ther Clin Risk Manag. 2006;2:129-58.
Mayr A, Aigner M, Lass-Flörl C. Caspofungin: When and how? The microbiologist’s view. Mycoses. 2011;55:27-35. https://doi.org/10.1111/j.1439-0507.2011.02039.x
Chen SCA, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs. 2011;71:11-41. https://doi.org/10.2165/11585270-000000000-00000
Lockhart SR, Zimbeck AJ, Baddley JW, Marr KA, Andes DR, Walsh TJ, et al. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the transplant-associated infection surveillance network. Antimicrob Agents Chemother. 2011;55:3944-6.
Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. Potency of anidulafungin compared to nine other antifungal agents tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: Results from the global sentry antimicrobial surveillance program (2008). J Clin Microbiol. 2010;48:2984-7. https://doi.org/10.1128/JCM.00328-10
Badali H, Vaezi A, Haghani I, Yazdanparast SA, Hedayati MT, Mousavi B, et al. Environmental study of azole-resistant Aspergillus fumigatus with TR34 /L98H mutations in the cyp51A gene in Iran. Mycoses. 2013;56:659-63. https://doi.org/10.1111/myc.12089
Faria-Ramos I, Farinha S, Neves-Maia J, Tavares PR, Miranda IM, Estevinho LM, et al. Development of cross-resistance by Aspergillus fumigatus to clinical azoles following exposure to prochloraz, an agricultural azole. BMC Microbiol. 2014;14:1-5. https://doi.org/10.1186/1471-2180-14-155
Lopetegui CM, Menéndez GMM, Pérez YT, López ME, Castillo EM. Aplicaciones de la aerobiología en el sistema de predicción y vigilancia de la enfermedad moho azul del tabaco en la provincia Pinar del Río, Cuba. Avances. 2006;8:1-11.
Monteil MA. Saharan dust clouds and human health in the English-speaking Caribbean: What we know and don’t know. Environ Geochem Health. 2008;30:339-43.
Some similar items:
- José Camilo Álvarez-Rodríguez, María Paula Blanco-Bustos, Sonia Isabel Cuervo-Maldonado, Julio César Gómez-Rincón , Ángela Reyes , Geotrichosis: fungemia in a patient with acute lymphoblastic leukemia , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Leidy González, Jorge Alberto Cortés, Systematic review of antimicrobial resistance in Enterobacteriaceae isolates from Colombian hospitals , Biomedica: Vol. 34 No. 2 (2014)
- Ana María García, Orville Hernández, Beatriz H. Aristizábal, Luz Elena Cano, Juan G. McEwen, Ángela Restrepo, Identification of genes associated with germination of conidia to form mycelia in the fungus Paracoccidioides brasiliensis , Biomedica: Vol. 29 No. 3 (2009)
- Juan Manuel Senior, Clara Saldarriaga, Endocarditis due to infection by Paecilomyces variotii , Biomedica: Vol. 29 No. 2 (2009)
- María Consuelo Garzón, Dailyn Yorledy Angée, Claudia Llerena, Dora Leticia Orjuela, Jorge Ernesto Victoria, Surveillance of Mycobacterium tuberculosis resistance to antituberculosis drugs , Biomedica: Vol. 28 No. 3 (2008)
- Silvia Blair, Eliana Arango, Jaime Carmona Fonseca, In vitro susceptibility of Colombian Plasmodium falciparum isolates to different antimalarial drugs , Biomedica: Vol. 28 No. 2 (2008)
- César A. Arias, Marylin Hidalgo, Jinnethe Reyes, Ana María Cárdenas, Lorena Díaz, Sandra Ríncon, Natasha Vanegas, Paula Lucía Díaz, Elizabeth Castañeda, Resistance profiles to fluoroquinolones in clinical isolates of Gram positive cocci , Biomedica: Vol. 28 No. 2 (2008)
- Ana María Perilla, Camilo González, Sandra Liliana Valderrama, Natasha Vanegas, Bibiana Chavarro, Luis Carlos Triana, José Roberto Támara, Carlos Arturo Álvarez, Necrotizing pneumonia by community-acquired, methicillin-resistant Staphylococcus aureus in Colombia , Biomedica: Vol. 29 No. 4 (2009)
- Juan D. Zapata, Diego H. Cáceres, Luz E. Cano, Catalina de Bedout, Sinar D. Granada, Tonny W. Naranjo, Standardization and validation of a high-efficiency liquid chromatography with a diode-array detector (HPLC-DAD) for voriconazole blood level determination , Biomedica: Vol. 44 No. 1 (2024)
- Claudia Llerena, Santiago Elías Fadul, María Consuelo Garzón, Graciela Mejía, Dora Leticia Orjuela, Luz Mary García, Hilda Beatriz Álvarez, Fernando Javier Ruiz, Drug-resistant Mycobacterium tuberculosis in children under 15 years , Biomedica: Vol. 30 No. 3 (2010)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























