Abundance, composition and natural infection of Anopheles mosquitoes from two malaria-endemic regions of Colombia
Abstract
Introduction: In Colombia there are three Anopheles species implicated in malaria transmission as primary vectors; however, the local role of some Anopheles species must still be defined.
Objective: To determine the abundance, composition and natural infection rates for Anopheles mosquitoes with Plasmodium spp. in two malaria-endemic regions of Colombia.
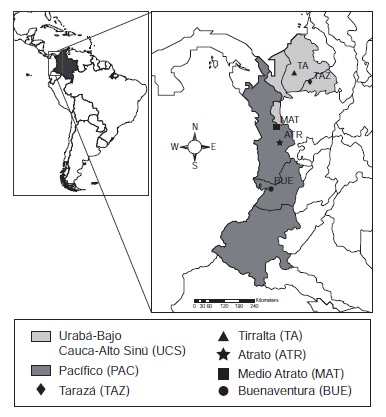
Materials and methods: Anopheles mosquitoes were collected using the human-landing catches and while resting in livestock corrals in nine localities of two malaria-endemic regions of Colombia. Mosquitoes were morphologically identified and confirmed by PCR-RFLP-ITS2. Identified mosquitoes were processed and tested for Plasmodium parasite infection by ELISA and ssrRNA-based nested PCR.
Results: We collected 1,963 Anopheles mosquitoes corresponding to nine species. The most abundant species were Anopheles nuneztovari (53.5%) and A. darlingi (34.5%), followed by A. triannulatus s.l. (6%), and other species (≈5.9%). Three species were naturally infected with Plasmodium spp.: A. darlingi, A. nuneztovari and A. triannulatus s.l.
Conclusions: Natural infection of A. darlingi and A. nuneztovari indicate that these malaria vectors continue to be effective carriers of Plasmodium in the localities under study in Valle del Cauca and Chocó. Additionally, the infected A. triannulatus s.l. collected in livestock corrals in the locality of the department of Córdoba suggests the need for further studies to define the epidemiological importance of this species given its abundance and opportunistic anthropophilic behavior.
Downloads
References
World Health Organization. World Malaria Report. Geneva: WHO; 2015. Accessed: June 25, 2016. Available from: http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf
Instituto Nacional de Salud. Comportamiento de los eventos de vigilancia en salud pública. Enfermedades transmitidas por vectores. Malaria. Semana epidemiológica número 52 de 2015 (27 dic. - 02 ene.). Boletín Epidemiológico Semanal. 2015;52. Accessed: March 3, 2017. Available from: http://www.ins.gov.co/boletin-epidemiologico/Boletn%20Epidemiolgico/2015%20Boletin%20epidemiologico%20Semana%2052.pdf
González R, Carrejo N. Introducción al estudio taxonómico de Anopheles de Colombia. Claves y notas de distribución. Segunda edición. Cali: Universidad del Valle; 2009. p. 260.
Montoya-Lerma J, Solarte YA, Giraldo-Calderón GI, Quiñones ML, Ruiz-López F, Wilkerson RC, et al. Malaria vector species in Colombia - A review. Mem Inst Oswaldo Cruz. 2011;106(Suppl.1):223-38. https://doi.org/10.1590/S0074-02762011000900028
Olano VA, Brochero HL, Sáenz R, Quiñones ML, Molina JA. Mapas preliminares de la distribución de especies de Anopheles vectores de malaria en Colombia. Biomédica. 2001;21:402-8.
Naranjo-Díaz N, Rosero D, Rua-Uribe G, Luckhart S, Correa M. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors. 2013;6:61. https://doi.org/10.1186/1756-3305-6-61
Naranjo-Díaz N, Altamiranda M, Luckhart S, Conn JE, Correa MM. Malaria vectors in ecologically heterogeneous localities of the Colombian Pacific region. PLoS One. 2014;9: e103769. https://doi.org/10.1371/journal.pone.0103769
Quiñones ML, Ruiz F, Calle DA, Harbach RE, Erazo HF, Linton Y. Incrimination of Anopheles (Nyssorhynchus) rangeli and An. (Nys.) oswaldoi as natural vectors of Plasmodium vivax in Southern Colombia. Mem Inst Oswaldo Cruz. 2006;101: 617-23. https://doi.org/10.1590/S0074-02762006000600007
Gómez GF, Bickersmith SA, González R, Conn JE, Correa MM. Molecular taxonomy provides new insights into Anopheles species of the Neotropical Arribalzagia series. PLoS One. 2015;10:e0119488. https://doi.org/:10.1371/journal.pone.0119488
Orjuela LI, Herrera M, Erazo H, Quiñones ML. Especies de Anopheles presentes en el departamento del Putumayo y su infección natural con Plasmodium. Biomédica. 2013;33: 42-52. https://doi.org/10.7705/biomedica.v33i1.619
Rosero DA, Naranjo-Díaz N, Álvarez N, Cienfuegos AV, Torres C, Luckhart S, et al. Colombian Anopheles triannulatus (Diptera: Culicidae) naturally infected with Plasmodium spp. ISRN Parasitol. 2013;2013:927453. https://doi.org/10.5402/2013/927453
Orjuela LI, Ahumada ML, Ávila I, Herrera S, Beier JC, Quiñones ML. Human biting activity, spatial-temporal distri-bution and malaria vector role of Anopheles calderoni in the southwest of Colombia. Malar J. 2015;14:256. https://doi.org/10.1186/s12936-015-0764-6
Instituto Geográfico Agustín Codazzi. Atlas de Colombia. Quinta edición. Bogotá: Imprenta Nacional de Colombia; 2002. p. 342.
Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbio-geographic and cladistic analyses of the entomofauna. Annu Rev Entomol. 2006;51:467-94. https://doi.org/10.1146/annurev.ento.50.071803.130447
Gutiérrez LA, Naranjo N, Jaramillo LM, Muskus C, Luckhart S, Conn JE, et al. Natural infectivity of Anopheles species from the Pacific and Atlantic Regions of Colombia. Acta Trop. 2008;107:99-105. https://doi.org/10.1016/j.actatropica.2008.04.019
Gutiérrez LA, González JJ, Gómez GF, Castro MI, Rosero DA, Luckhart S, et al. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in the states of Córdoba and Antioquia, Northwestern Colombia. Mem Inst Oswaldo Cruz. 2009;104:1117-24. https://doi.org/10.1590/S0074-02762009000800008
Zapata MA, Cienfuegos AV, Quirós OI, Quiñones ML, Luckhart S, Correa MM. Discrimination of seven Anopheles species from San Pedro de Urabá, Antioquia, Colombia, by polymerase chain reaction-restriction fragment length polymorphism analysis of ITS sequences. Am J Trop Med Hyg. 2007;77:67-72. https://doi.org/10.4269/ajtmh.2007.77.67
Cienfuegos AV, Gómez GF, Córdoba LA, Luckhart S, Conn JE, Correa MM. Diseño y evaluación de metodolo-gías basadas en PCR-RFLP de ITS2 para la identificación molecular de mosquitos Anopheles spp. (Diptera: Culicidae) de la Costa Pacífica de Colombia. Rev Biomed. 2008;19: 35-44.
Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol.1987;24:433-7.
Wirtz RA, Charoenvit Y, Burkot TR, Esser KM, Beaudoin RL, et al. Evaluation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med Vet Entomol. 1991;5:17-22. https://doi.org/10.1111/j.1365-2915.1991.tb00515.x
Wirtz RA, Sattabongkot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immu-nosorbent assay for Plasmodium vivax-VK247 sporozoites. J Med Entomol. 1992;29:854-7.
Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65: 39-45.
Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epide-miologic studies. Am J Trop Med Hyg. 1999;60:687-92.
Rosero DA, Gutiérrez LA, Cienfuegos AV, Jaramillo LM, Correa MM. Optimización de un procedimiento de extracción de ADN para mosquitos anofelinos. Rev Colomb Entomol. 2010;36:260-3.
Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: What makes a malaria vector? Trends Parasitol. 2010;26:130-6. https://doi.org/10.1016/j.pt.2009. 12.001
Sierra DM, Vélez ID, Linton YM. Malaria vector Anopheles (Nyssorhynchus) nuneztovari comprises one genetic species in Colombia based on homogeneity of nuclear ITS2 rDNA. J Med Entomol. 2004;41:302-7. https://doi.org/10.1603/0022-2585-41.3.302
González R, Martínez LM. Nuevo registro de distribución altitudinal de Anopheles albimanus Wiedemann (Diptera: Culicidae). Boletín del Museo de Entomología. 2006;7:19-23.
Pinault LL, Hunter FF. New highland distribution records of multiple Anopheles species in the Ecuadorian Andes. Malar J. 2011;10:236. https://doi.org/10.1186/1475-2875-10-236.
Olano VA, Carrasquilla G, Méndez F. Transmisión de malaria urbana en Buenaventura, Colombia: aspectos ento-mológicos. Pan Am. Public Health. 1997;1:287-94. https://doi.org/10.1590/S1020-49891997000400005
Conde M, Pareja P, Orjuela L, Ahumada M, Durán S, Jara JA, et al. Larval habitat characteristics of the main malaria vectors in the most endemic regions of Colombia: Potential implications for larval control. Malar J. 2015;14:476. https://doi.org/10.1186/s12936-015-1002-y
Aycardi-Morinelli MP, Correa-Ochoa MM, Zárate-Peñata EdC, Padrón-Echenique C. Determinación de especies anofelinas en una localidad endémica de malaria en el depar-tamento de Córdoba, noroeste de Colombia. Entomotropica. 2016;31:294-301.
Some similar items:
- Pilar Jiménez, Jan E. Conn, Robert Wirtz, Helena Brochero, Anopheles (Díptera: Culicidae) vectors of malaria in Puerto Carreño municipality, Vichada, Colombia , Biomedica: Vol. 32 (2012): Suplemento 1, Malaria
- Andrés López-Rubio, Juan David Suaza, Charles Porter, Sandra Uribe, Gabriel Bedoya, Iván Darío Vélez, Phylogenetic signal at the Cytb-SertRNA-IG1-ND1 mitochondrial region in Anopheles (Kerteszia) neivai Howard, Dyar & Knab, 1913 , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Lorena I. Orjuela, Manuela Herrera, Holmes Erazo, Martha L. Quiñones, Anopheles species present in the department of Putumayo and their natural infectivity with Plasmodium , Biomedica: Vol. 33 No. 1 (2013)
- Helena Brochero, Paula Ximena Pareja, Gloria Ortiz, Víctor Alberto Olano, Breeding places and biting activity of Anopheles species in the municipality of Cimitarra, Santander, Colombia. , Biomedica: Vol. 26 No. 2 (2006)
- Lyda Osorio, Ligia del Pilar Pérez, Iveth J. González, Assessment of the efficacy of antimalarial drugs in Tarapacá, in the Colombian Amazon basin , Biomedica: Vol. 27 No. 1 (2007)
- José Joaquín Carvajal, Ligia Inés Moncada, Mauricio Humberto Rodríguez, Ligia del Pilar Pérez, Víctor Alberto Olano, Characterization of Aedes albopictus (Skuse, 1894) (Diptera:Culicidae) larval habitats near the Amazon River in Colombia , Biomedica: Vol. 29 No. 3 (2009)
- María Elena Cuéllar-Jiménez, Olga Lucía Velásquez-Escobar, Ranulfo González-Obando, Carlos Andrés Morales-Reichmann, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia , Biomedica: Vol. 27 No. 2 (2007)
- Larry Niño, Use of the function semivariogram and kriging estimation in the spacial analysis of Aedes aegypti (Diptera: Culicidae) distributions , Biomedica: Vol. 28 No. 4 (2008)
- Ronald Maestre, Consuelo Vergara Sanchez, Guillermo Berrueco Rodríguez, Betsy Bello Novoa, Helena Brochero, Presence of Haemagogus equinus Theobald, 1903 (Diptera: Culicidae), in Soledad and Malambo, in the Province of Atlántico, Colombia , Biomedica: Vol. 28 No. 1 (2008)
- Rosa Magdalena Uscátegui, Adriana M. Correa, Jaime Carmona-Fonseca, Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria , Biomedica: Vol. 29 No. 2 (2009)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























