A new theory of depression based on the serotonin/kynurenine relationship and the hypothalamicpituitary- adrenal axis
Abstract
The serotonergic and immunological hypothesis of depression proposes that certain types of excessive stress distort the relationship between the activities of the innate immune and central nervous systems, so that the stress caused by an infection, or excessive psychological stress, activate toll-like receptors such as the TLR-4, the transcription factor NF-kB, the inflammasome NLRP3, as well as the secretion of interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and other factors of the innate immune response, causing first, the general symptoms of the disease which appear with any infection, but also those characteristic of depressive illness such as dysphoria and anhedonia.
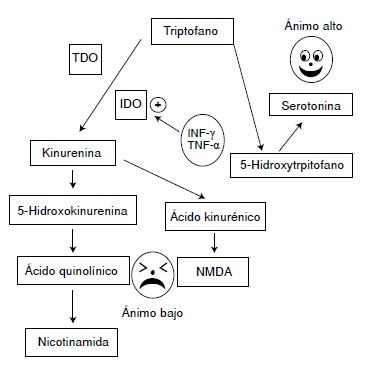
The evidence indicates that, if the stimulus persists or recurs within 24 hours, the indole-2, 3-dioxygenase enzyme (IDO) of the kynurenine metabolic pathway, which increases the synthesis of quinolinic acid, is activated with an associated reduction of serotonin synthesis. Quinolinic acid activates NMDA receptors in the central nervous system and stimulates the secretion of interleukins IL-6 and 1L-1β, among others, promoting hyper-activity of the HPA axis and reinforcing a bias of the tryptophan metabolism to produce quinolinic acid, and interleukins by the innate immune system, further reducing the synthesis of serotonin and consolidating the depressive process.
We discuss the evidence showing that this process can be initiated by either interleukin stimulated by an infection or some vaccines or excessive psychological stress that activates the HPA axis together with said innate immune response, causing a process of aseptic inflammation in the central nervous system.
Downloads
References
Sankowski R, Mader S, Valdes-Ferrer SI. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28. https://doi.org/10.3389/fncel.2015.00028
Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98-101. https://doi.org/10.1126/science.1209985
Medzhitov R, Janeway CA Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell. 1997; 91:295-8. https://doi.org/10.1016/S0092-8674(00)80412-2
Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323-9. https://doi.org/10.1038/nature05286
Aballay A. Neural regulation of immunity: Role of NPR-1 in pathogen avoidance and regulation of innate immunity. Cell Cycle. 2009;8:966-9. https://doi.org/10.1016/S0960-9822(02)01396-9
Aballay A, Drenkard E, Hilbun LR, Ausubel FM. Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol. 2003;13:47-52. https://doi.org/10.1016/S0960-9822(02)01396-9
Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83-107. https://doi.org/10.1037/0033-295X.105.1.83
Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, et al. Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharma cology. 1999;20:370-9. https://doi.org/10.1016/S0893-133X(98)00088-8
Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:630-3. https://doi.org/10.1176/ajp.2006.163.9.1630
Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298-306. https://doi.org/10.1016/0306-9877(91)90266-2
Hernández JC, Montoya CJ, Urcuqui-Inchima S. The role of toll-like receptors in viral infections: HIV-1 as a model. Biomédica. 2007;27:280-93. https://doi.org/10.7705/biomedica.v27i2.225
Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111-6. https://doi.org/10.1016/0306-4530(94)00066-J
Schiepers, OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201-17. https://doi.org/10.1016/j.pnpbp.2004.11.003
Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology
and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643-52.https://doi.org/S0893-133X(01)00407-9
Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: Evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050-7.
Ramírez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressivelike behaviors. Brain Behav Immun. 2016;57:293-303. https://doi.org/10.1016/j.bbi.2016.05.008
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24-31. https://doi.org/10.1016/j.it.2005.11.006
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56. https://doi.org/10.1038/nrn2297
Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, et al. Inflammation and neuronal plasticity: A link between childhood trauma and depression pathogenesis. Front Cell Neurosci. 2015;9:40. https://doi.org/10.3389/fncel.2015.00040
Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426-36.
https://doi.org/ 10.1016/j.psyneuen.2010.09.012
O’Brien SM, Scott LV, Dinan TG. Cytokines: Abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19:397-403.
https://doi.org/doi/10.1002/hup.609
Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav Immun. 2001;15:7-24. https://doi.org/10.1006/brbi.2000.0613
Leonard BE. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl.3):S302-6. https://doi.org/10.1016/S0924-
(05)80180-4
González-Peña D, Nixon SE, O’Connor JC, Southey BR, Lawson MA, McCusker RH, et al. Microglia transcriptome changes in a model of depressive behavior after immune challenge. PLoS One. 2016;11:e0150858. https://doi.org/10.1371/journal.pone.0150858
Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586-90. https://doi.org/10.1111/j.1749-6632.1998.tb09597.x
Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martínez J, Furness L, et al. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283-
https://doi.org/10.1016/0006-8993(94)91742-6
Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1 beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281-93. https://doi.org/10.1016/S0306-4522(97)00350-3
Hasegawa-Ishii S, Inaba M, Umegaki H, Unno K, Wakabayashi K, Shimada A. Endotoxemia-induced cytokine-mediated responses of hippocampal astrocytes transmitted by cells of the brain-immune interface. Sci Rep. 2016;6:254-57. https://doi.org/10.1038/srep25457
Banks WA. The blood-brain barrier in psychoneuroimmunology. Neurol Clin. 2006;24:413-9. https://doi.org/10.1016/j.ncl.2006.03.009
Konsman J P, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: Relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113-29. https://doi.org/10.1002/cne.20052
Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: Parallel or convergent? Auton Neurosci. 2000;85:60-5. https://doi.org/10.1016/S1566-0702(00)00220-4
Lenart N, Brough D, Denes A. Inflammasomes link vascular disease with neuroinflammation and brain disorders. J Cereb Blood Flow Metab. 2016;36:1668-85.
https://doi.org/10.1177/0271678X16662043
Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897-913. https://doi.org/10.1002/jnr.23550
García-Oscos F, Peña D, Housini M, Cheng D, López D, Cuevas-Olguín R, et al. Activation of the anti-inflammatory reflex blocks lipopolysaccharide-induced decrease in synaptic inhibition in the temporal cortex of the rat. J Neurosci Res. 2015;93:859-65. https://doi.org/10.1002/jnr.23550
García-Oscos F, Peña D, Housini M, Cheng D, López D, Borland MS, et al. Vagal nerve stimulation blocks interleukin 6-dependent synaptic hyperexcitability induced by lipopolysaccharide-induced acute stress in the rodent prefrontal cortex. Brain Behav Immun. 2015;43:149-58. https://doi.org/10.1016/j.bbi.2014.07.020
Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5-14. https://doi.org/10.1016/S0165-5728(02)00022-X
Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav. 2016:161:166-73. https://doi.org/10.1016/j.physbeh.2016.04.034
Vogelzangs N, de Jonge P, Smit JH, Bahn S. Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6:e825. https://doi.org/10.1038/tp.2016.92
Salgado H, García-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, et al. Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex. 2011;21:212-21. https://doi.org/10.1093/cercor/bhq081
Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111-5. https://doi.org/10.1038/35040600
Watters TM, Kenny EF, O’Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85:411-9. https://doi.org/10.1038/sj.icb.7100095
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499-511. https://doi.org/10.1038/nri1391
Cheng Y, Pardo M, Armini R de S, Martínez A, Mouhsine H, Zagury JF, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207-22. https://doi.org/10.1016/j.bbi.2015.12.012
McCusker RH, Kelley KW. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216:84-98. https://doi.org/10.1242/jeb.073411
Viviani B, Boraso M, Marchetti N, Marinovich M. Perspectives on neuroinflammation and excitotoxicity: A neurotoxic conspiracy? Neurotoxicology. 2014;43:10-20.
https://doi.org/10.1016/j.neuro.2014.03.004
Yeh KY, Shou SS, Lin Y X, Chen C C, Chiang C Y, Yeh CY. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother Res. 2015;29:260-6. https://doi.org/10.1002/ptr.5247
Remus JL, Dantzer R. Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. Int J Neuropsychopharmacol. 2016;19:9. https://doi.org/10.1093/ijnp/pyw028
Wichers MC, Maes M. The role of indoleamine 2,3- dioxygenase (IDO) in the pathophysiology of interferonalpha- induced depression. J Psychiatry Neurosci. 2004;29:11-7.
Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol Psychiatry. 2007;12:988-1000. https://doi.org/10.1038/sj.mp.4002006
Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105-23. https://doi.org/10.1016/j.brainresrev.2009.05.005
O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200-9. https://doi.org/10.1523/
JNEUROSCI.5032-08.2009
Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722-9. https://doi.org/10.1016/j.pnpbp.2010.04.011
Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596-601. https://doi.org/10.1016/S0889-1591(02)00014-4
Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516-31. https://doi.
org/10.1016/j.psyneuen.2007.03.005
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressivelike behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511-22. https://doi.org/10.1038/sj.mp.4002148
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609-16. https://doi.org/10.1038/npp.2013.71
Gibney SM, McGuinness B, Prendergast C, Harkin A, Connor TJ. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. 2013;28:170-81. https://doi.org/10.1016/j.bbi.2012.11.010
Fischer CW, Elfving B, Lund S, Wegener G. Behavioral and systemic consequences of long-term inflammatory challenge. J Neuroimmunol. 2015;288:40-6. https://doi.
org/10.1016/j.jneuroim.2015.08.011
O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressivelike behavior. J Immunol. 2009;182:3202-12. https://doi.org/10.4049/jimmunol.0802722
Miller GE, Chen E, Sze J, Marin T, Arévalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266-72. https://doi.org/10.1016/j.biopsych.2008.03.017
Hayden MS, West AP, Ghosh S. SnapShot: NF-kappaB signaling pathways. Cell. 2006;127:1286-7. https://doi.org/10.1016/j.cell.2006.12.005
Muller MB, Holsboer F. Mice with mutations in the HPAsystem as models for symptoms of depression. Biol Psychiatry. 2006;59:1104-15. https://doi.org/10.1016/j.biopsych.2006.02.008
Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86-105. https://doi.org/10.1111/j.1749-6632.2009.04984.x
Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169-73.
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397-409. https://doi.org/10.1038/nrn2665
Littrell JL. Taking the perspective that a depressive state reflects inflammation: Implications for the use of antidepressants. Front Psychol. 2012;3:297. https://doi.org/10.3389/fpsyg.2012.00297
Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1beta: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939-49. https://doi.org/10.1038/npp.2011.277
Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391-404. https://doi.org/10.1016/S0006-3223(00)01088-X
Hennessy MB, Kaiser S, Sachser N. Stability and change: Stress responses and the shaping of behavioral phenotypes over the life span. Front Zool. 2015;12(Suppl.1):S18. https://doi.org/10.1186/1742-9994-12-S1-S18
Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do earlylife events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149-64. https://doi.org/10.1016/S0736-5748(98)00025-2
Kanczkowski W, Alexaki VI, Tran N, Grossklaus S, Zacharowski K, Martinez A, et al. Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci U S A. 2013;110:14801-6. https://doi.org/10.1073/pnas.1313945110
Mohn CE, Fernández-Solari J, De Laurentiis A, Bornstein SR, Ehrhart-Bornstein M, Rettori V. Adrenal gland responses to lipopolysaccharide after stress and ethanol administration in male rats. Stress. 2011;14:216-26. https://doi.org/10.3109/10253890.2010.532254
Loum-Ribot E, Lafon P, Chaigniau M, Tramu G, Corio M. Glucocorticoids down-regulate lipopolysaccharide-induced de novo production of neurotensin mRNA in the rat hypothalamic, paraventricular, corticotrophin-releasing hormone neurons. Neuroimmunomodulation, 2006;13:170-8. https://doi.org/10.1159/000098130
Gibb J, Al-Yawer F, Anisman H. Synergistic and antagonistic actions of acute or chronic social stressors and an endotoxin challenge vary over time following the challenge. Brain Behav Immun. 2013;28:149-58. https://doi.org/10.1159/000098130
Oliveira JF, Gomes CA, Vaz SH, Sousa N, Pinto L. Glial plasticity in depression. Front Cell Neurosci. 2016;10:163. https://doi.org/10.3389/fncel.2016.00163
Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 200;64:863-70. https://doi.org/10.1016/j.biopsych.2008.06.008
Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773-7. https://doi.org/ 10.1038/nm.3162
Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305-20. https://doi.org/10.1038/npp.2011.319
Di Benedetto B, Malik VA, Begum S, Jablonowski L, Gómez-González GB, Neumann ID, et al. Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity
of astrocyte processes. Front Cell Neurosci. 2016;10:8. https://doi.org/10.3389/fncel.2016.00008
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616-26. https://doi.org/10.1038/sj.npp.1300982
Etievant A, Lucas G, Dkhissi-Benyahya O, Haddjeri N. The role of astroglia in the antidepressant action of deep brain stimulation. Front Cell Neurosci. 2016;9:509. https://doi.org/10.3389/fncel.2015.00509
Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, et al. Requirement of AQP4 for antidepressive efficiency of fluoxetine: Implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263-76. https://doi.org/10.1038/npp.2008.185
Rial D, Lemos C, Pinheiro H, Duarte JM, Goncalves FQ, Real JI, et al. Depression as a glial-based synaptic dysfunction. Front Cell Neurosci. 2015;9:521. https://doi.org/10.3389/fncel.2015.00521
Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397-400. https://doi.org/10.1126/science.4035356
Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421-34. https://doi.org/10.1038/nrn3231
Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci Biobehav Rev. 2010;35:39-45. https://doi.org/10.1016/j.neubiorev.2010.01.003
Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci. 2013;1:331-48.
Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial proinflammatory responses. Brain Behav Immun. 2012;26:337-45. https://doi.org/10.1016/j.bbi.2011.10.005
Zacharowski K, Zacharowski PA, Koch A, Baban A,Tran N, Berkels R, et al. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci USA. 2006;103:6392-7. https://doi.org/10.1073/pnas.0601527103
Hernández ME, Mendieta D, Pérez-Tapia M, Bojalil R, Estrada-García I, Estrada-Parra S, et al. Effect of selective serotonin reuptake inhibitors and immunomodulator on cytokines levels: An alternative therapy for patients with major depressive disorder. Clin Dev Immunol. 2013;2013:267871. https://doi.org/10.1155/2013/267871
Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez M A, Garcés-Álvarez M E, Hurtado-Alvarado G, et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. https://doi.org/10.1155/2015/354957
Some similar items:
- Wbeimar Aguilar-Jiménez, Wildeman Zapata, María Teresa Rugeles, Differential expression of human beta defensins in placenta and detection of allelic variants in the DEFB1 gene from HIV-1 positive mothers , Biomedica: Vol. 31 No. 1 (2011)
- Ana María Mejía-Jaramillo, Geysson Javier Fernández, Marleny Montilla, Rubén Santiago Nicholls, Omar Triana-Chávez, Trypanosoma cruzi strains resistant to benznidazole occurring in Colombia , Biomedica: Vol. 32 No. 2 (2012)
- Jorge A. Vega, Simón Villegas-Ospina, Wbeimar Aguilar-Jiménez, María T. Rugeles, Gabriel Bedoya, Wildeman Zapata, Haplotypes in CCR5-CCR2, CCL3 and CCL5 are associated with natural resistance to HIV-1 infection in a Colombian cohort , Biomedica: Vol. 37 No. 2 (2017)
- Jean Paul Vernot, Hernando del Castillo, Characterizing the CD3 epsilon chain from the New World primate Aotus nancymaae , Biomedica: Vol. 28 No. 2 (2008)
- Juan Carlos Hernández, Carlos Julio Montoya, Silvio Urcuqui-Inchima, The role of toll-like receptors in viral infections: HIV-1 as a model , Biomedica: Vol. 27 No. 2 (2007)
- Olga María Moreno, Clara Isabel González, Diego Luis Saaibi, William Otero, Reynaldo Badillo, Javier Martín, Gerardo Ramírez, Polymorphisms of IL-10 gene promoter and rheumatoid arthritis in a Colombian population , Biomedica: Vol. 27 No. 1 (2007)
- Lukas Tamayo-Orrego, Orlando Torres-Fernández, Protocol for the combination of neurohistological techniques on vibratome obtained sections , Biomedica: Vol. 31 No. 3 (2011)
- Nora Cardona-Castro, Miryan Sánchez-Jiménez, Winston Rojas, Gabriel Bedoya-Berrío, IL-10 gene promoter polymorphisms and leprosy in a Colombian population sample , Biomedica: Vol. 32 No. 1 (2012)
- Elizabeth García, Silvia Duarte, Camila Calderón, John Mario González, Adriana Cuéllar, Alberto Gómez, Evelyne Halpert, Adriana Rodríguez, Expression of IL-10, IL-4 and IFN-γ in active skin lesions of children with papular urticaria , Biomedica: Vol. 31 No. 4 (2011)
- Ana Victoria Valencia, Ana Lucía Páez, María Elena Sampedro, Clara Ávila, Julio Cesar Cardona, Catalina Mesa, Lina Galvis, Jaime Carrizosa, Mauricio Camargo, Andrés Ruíz, William Cornejo, Gabriel Bedoya, Evidence for association and epistasis between the genetic markers SLC6A4 and HTR2A in autism etiology , Biomedica: Vol. 32 No. 4 (2012)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























