Neuroanatomical evidence of the transport of the rabies virus through the propriospinal tract in the spinal cord of mice
Abstract
Introduction: Information about the neuroanatomical details of the ascendant transport of the rabies virus through the spinal cord is scarce.
Objective: To identify the neuroanatomical route of dissemination of the rabies virus at each of the levels of the spinal cord of mice after being inoculated intramuscularly.
Materials and methods: Mice were inoculated with the rabies virus in the hamstrings. After 24 hours post-inoculation, every eight hours, five animals were sacrificed by perfusion with paraformaldehyde. Then, the spinal cord was removed, and transverse cuts were made at the lumbosacral, thoracic, and cervical levels. These were processed by immunohistochemistry for the detection of viral antigens.
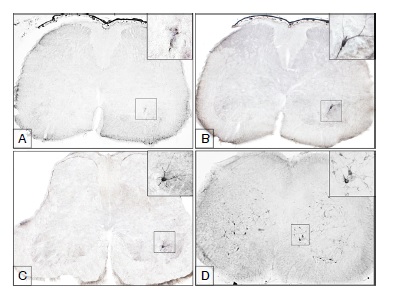
Results: The first antigens of rabies were observed as aggregated particles in the lumbar spinal cord at 24 hours post-inoculation, within the ventral horn in the same side of the inoculated limb. At 32 hours post inoculation the first motoneurons immunoreactive to the virus became visible. At 40 hours postinoculation the first immunoreactive neurons were revealed in the thoracic level, located on lamina 8 and at 48 hours post-inoculation in the cervical cord, also on lamina 8. At 56 hours post-inoculation the virus had spread throughout the spinal cord, but the animals still did not show signs of the disease.
Conclusion: In the mouse model we used, the rabies virus entered the spinal cord through the motoneurons and probably used the descending propriospinal pathway for its retrograde axonal transport to the encephalus.
Downloads
References
Murphy FA. Rabies pathogenesis. Brief review. Arch Virol. 1977;54:279-97. https://doi.org/10.1007/BF01314774
Jackson AC, Zhen F. Pathogenesis. En: Jackson AC, editor. Rabies. Tercera edición. San Diego: Academic Press; 2013. p. 299-349.
Kristensson K. Retrograde transport of macromolecules in axons. Annu Rev Pharmacol Toxicol. 1978;18:97-110. https://doi.org/10.1146/annurev.pa.18.040178.000525
Tsiang H. Evidence for an intraaxonal transport of fixed and street rabies virus. J Neuropathol Exp Neurol. 1979;38:286-99. https://doi.org/10.1097/00005072-197905000-00008
Watson HD, Tignor GH, Smith AL. Entry of rabies virus into peripheral nerves mice. J Gen Virol. 1981;56:371-82. https://doi.org/10.1099/0022-1317-56-2-371
Gillet JP, Derer P, Tsiang H. Axonal transport of rabies virus in the central nervous system of the rat. J Neuropathol Exp Neurol. 1986;45:619-34.
Ugolini G. Rabies virus as a transneural tracer of neural connections. Adv Virus Res. 2011;79:165-202. https://doi.org/10.1016/B978-0-12-387040-7.00010-X
World Health Organization. WHO Expert consultation on rabies. En: Technical Report Series No. 982. Geneva: WHO Press; 2013. p. 1-139.
Tsiang H, Lycke E, Ceccaldi PE, Ermine A, Hirardot X. The anterograde transport of rabies virus in rat sensory dorsal root ganglia neurons. J Gen Virol. 1989;70:2075-85. https://doi.org/10.1099/0022-1317-70-8-2075
Castellanos J, Hurtado H, Arias J, Velandia A. Rabies virus infection of cultured adult mouse dorsal root ganglion neurons. Mem Inst Oswaldo Cruz. 1996;91:621-5. https://doi.org/10.1590/S0074-02761996000500014
Velandia-Romero ML, Castellanos JE, Martínez-Gutiérrez M. In vivo differential susceptibility of sensory neurons to rabies virus infection. J Neurovirol. 2013;13:367-75. https://doi.org/10.1007/s13365-013-0179-5
Coulon P, Derbin C, Kucera P, Lafay F, Prehaud C, Flamand A. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J Virol. 1989;63:3550-4.
Zampieri N, Jessell TM, Murray AJ. Mapping sensory circuits by anterograde trans-synaptic transfer of recombinant rabies virus. Neuron. 2014;81:766-78. https://doi.org/10.1016/j.neuron.2013.12.033
Haberl MG, Viana da Silva S, Guest JM, Ginger M, Ghanem A, Mulle C, et al. An anterograde rabies virus vector for high-resolution large-scale reconstruction of 3D neuron morphology. Brain Struct Funct. 2015;220:1369-79. https://doi.org/10.1007/s00429-014-0730-z
Heise C, Kayalioglu G. Cytoarchitecture of the spinal cord. En: Watson C, Paxinos G, Kayalioglu G, editores. The spinal cord. San Diego: Academic Press; 2009. p. 64-93.
Habel K. Habel Test For Potency. En: Meslin FX, Kaplan MKH, editores. Laboratory techniques in rabies. Cuarta edición. Geneva: WHO Press; 1996. p. 369-73.
Lamprea NP, Ortega LM, Santamaría G, Sarmiento L, Torres-Fernández O. Elaboración y evaluación de un antisuero para la detección inmunohistoquímica del virus de la rabia en tejido cerebral fijado en aldehídos. Biomédica. 2010;30:146-51. https://doi.org/10.7705/biomedica.v30i1.162
Watson C, Paxinos G, Kayalioglu G, Heise Claire. Atlas of the mouse spinal cord. En: Watson C, Paxinos G, Kayalioglu G, editores. The espinal cord. San Diego: Academic Press;2009. p. 308-79.
Sengul G. Watson C. Spinal Cord: En: Watson C, Paxinos G, Puelles L. The mouse nervous system. San Diego: Academic Press; 2012. p. 424-58.
McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Phil Trans R Soc Lond B. 1981;293:477-508. https://doi.org/10.1098/rstb.1981.0082
McHanwell S, Watson C. Localization of motoneurons in the spinal cord. En: Watson C, Paxinos G, Kayalioglu G, editores. The spinal cord. San Diego: Academic Press; 2009. p. 92-114.
Conta AC, Stelzner DJ. The propiospinal system. En: Watson C, Paxinos G, Kayalioglu G, editores. The spinal cord. San Diego: Academic Press; 2009. p. 178-90.
Ni Y, Nawabi H, Liu X, Yang L, Miyamichi K, Tedeschi A, et al. Characterization of long descending premotor propiospinal neurons in the spinal cord. J Neurosci. 2014;34:9404-17. https://doi.org/10.1523/JNEUROSCI.1771-14.2014
Juntrakul S, Ruangvejvorachai P, Shuangshoti S, Wacharapluesadee S, Hemachudha T. Mechanisms of scape phenomenon of spinal cord and brainstem in human rabies. BMC Infect Dis. 2005;5:104. https://doi.org/10.1186/1471-2334-5-104
Kojima D, Park CH, Satoh Y, Inoue S, Noguchi A, Oyamada T. Pathology of the spinal cord of C57BL/6J mice infected with rabies virus (CVS-11) strain. J Med Vet Sci. 2009;71:319-24. https://doi.org/10.1292/jvms.71.319
Bassuino DM, Konradt G, Cruz RA, Silva GS, Gomes DC, Pavarini SP, et al. Characterization of spinal cord lesions in cattle and horses with rabies: the importance of correct sampling. J Vet Diagn Invest. 2016;28:455-60. https://doi.org/10.1177/1040638716647992
Dean DJ, Evans WM, McClure RC. Pathogenesis of rabies. Bull World Health Organ. 1963;29:803-11.
Baer GM, Shanthaveerappa TR, Bourne GH. Studies on the pathogenesis of fixed rabies virus in rats. Bull World Health Organ. 1965;33:783-94.
Watson HD, Tignor GH, Smith AL. Entry of rabies virus into the peripheral nerves of mice. J Gen Virol. 1981;56:371-82. https://doi.org/10.1099/0022-1317-56-2-371
Tsiang H. Pathophysiology of rabies virus infection of the nervous system. Adv Virus Res.1993;42:375-412.
Ugolini G. Use of rabies virus as transneuronal tracer of neuronal connections: Implications for the understanding of rabies pathogenesis. Dev Biol (Basel). 2008;131:493-506.
Monroy-Gómez J, Torres-Fernández O. Distribución de calbindina y parvoalbúmina y efecto del virus de la rabia sobre su expresión en la médula espinal de ratones. Biomédica. 2013;33:564-73. https://doi.org/10.7705/biomedica.v33i4.1552
Lamprea N, Torres-Fernández O. Evaluación inmunohistoquímica de la expresión de calbindina en el cerebro de ratones en diferentes tiempos después de la inoculación con el virus de la rabia. Colomb Med. 2008;39(Supl.3):7-13.
Some similar items:
- Jeison Monroy-Gómez, Orlando Torres-Fernández, Calbindin and parvalbumin distribution in spinal cord of normal and rabies-infected mice , Biomedica: Vol. 33 No. 4 (2013)
- Edwin Abraham Medina, Middle ear adenoma , Biomedica: Vol. 29 No. 3 (2009)
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Felipe García, Martha C. Domínguez, Miyerlandi Torres, Óscar Tamayo, William Criollo, Milton Quintana, Adalberto Sánchez, Autoimmune syndrome in the tropical spastic paraparesis/myelopathy associated with human T-lymphotropic virus infections , Biomedica: Vol. 28 No. 4 (2008)
- Aura Caterine Rengifo, Orlando Torres-Fernández, Decreased number neurons expressing GABA in the cerebral cortex of rabies-infected mice , Biomedica: Vol. 27 No. 4 (2007)
- Nina Paola Lamprea, Lina María Ortega, Gerardo Santamaría, Ladys Sarmiento, Orlando Torres-Fernández, Production and evaluation of an antiserum for immunohistochemical detection of rabies virus in aldehyde fixed brain tissues , Biomedica: Vol. 30 No. 1 (2010)
- Orlando Torres-Fernández, Gloria E. Yepes, Javier E. Gómez, Neuronal dentritic morphology alterations in the cerebral cortex of rabies-infected mice: a Golgi study , Biomedica: Vol. 27 No. 4 (2007)
- Nadia Yadira Castañeda, Jacqueline Chaparro-Olaya, Jaime E. Castellanos, Production and characterization of a polyclonal antibody against rabies virus phosphoprotein , Biomedica: Vol. 27 No. 2 (2007)
- Jessika Valderrama, Ingrid García, Germán Figueroa, Edilberto Rico, Juliana Sanabria, Nicolás Rocha, Edgar Parra, Cecilia Saad, Andrés Páez, Outbreaks of human rabies transmitted by vampire bats in Alto Baudó and Bajo Baudó municipalities, department of Chocó, Colombia, 2004-2005 , Biomedica: Vol. 26 No. 3 (2006)
- Andrés F. Londoño, Silvana Levis, Juan D. Rodas, Hantavirus as important emerging agents in South America , Biomedica: Vol. 31 No. 3 (2011)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























