Drug utilization study of two generic antibiotics in a tertiary hospital in Bogotá
Abstract
Introduction: The Colombian national pharmaceutical policy establishes as a strategy the generation of greater pharmaco-epidemiological research at the national level, especially in the case of antibiotic drugs.
Objective: To provide local pharmaco-epidemiological evidence regarding the effectiveness, conditions of use and safety of generic meropenem and cefepime in a tertiary hospital in Bogotá.
Materials and methods: We conducted a descriptive, longitudinal and retrospective drug utilization study. The data were collected from the medical histories of all the patients who had cefepime or meropenem prescribed.
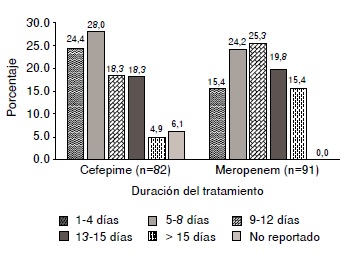
Results: We included 82 patients treated with cefepime and 91 treated with meropenem in the study. Most of the patients were in services different from the intensive care unit (taking cefepime: 59.8%, and meropenem: 52.7%). Only 21.9% of the patients treated with cefepime and 49% of those treated with meropenem were seen by an infectious disease specialist. The antibiogram was performed for 47% and 60% of the patients treated with cefepime and meropenem, respectively. The most frequent
indication for cefepime were respiratory infections and for meropenem, genitourinary ones. Therapeutic success rates were 61.7% for cefepime and 63.0% for meropenem.
Conclusions: This study contributes evidence regarding the therapeutic performance of two generic antibiotics used in tertiary hospitals. There were no reports of therapeutic failure during the study period. In the cases of non-response, pharmacokinetic alterations, unfavorable clinical conditions, and inappropriate choice of antimicrobial treatment were identified as frequent factors.
Downloads
References
Acosta A. Descripción del gasto y consumo de medicamentos biotecnológicos útiles en el tratamiento de artritis reumatoide refractaria: series temporales para Argentina, Colombia y Ecuador (tesis). Buenos Aires: Universidad de Buenos Aires; 2017.
World Health Organization. Global action plan on antimicrobial resistance. 1st edition. Geneva: WHO; 2015. p. 19.
Vesga O, Agudelo M, Salazar B, Rodríguez C, Zuluaga A. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob Agents Chemother. 2010;5:3271-9. https://doi.org/10.1128/AAC.01044-09
Rodríguez CA, Agudelo M, Aguilar YA, Zuluaga AF, Vesga O. Impact on bacterial resistance of therapeutically nonequivalent generics: The case of piperacillintazobactam. PLoS One. 2016;11:e0155806. https://doi.org/10.1371/journal.pone.0155806
Tattevin P, Saleh-Mghir A, Davido B, Ghout I, Massias L, García De La María C, et al. Comparison of six generic vancomycin products for treatment of methicillin-resistant Staphylococcus aureus experimental endocarditis in rabbits. Antimicrob Agents Chemother. 2013;57:1157-62. https://doi.org/10.1128/AAC.01669-12
Tattevin P, Crémieux AC, Rabaud C, Gauzit R. Efficacy and quality of antibacterial generic products approved for human use: A systematic review. Clin Infect Dis. 2014;58:458-69. https://doi.org/10.1093/cid/cit769
Pallares CJ, Martínez E. Factores de riesgo asociados a mortalidad en infecciones relacionadas con la atención en salud en un hospital universitario de tercer nivel en Colombia. Biomédica. 2014;34:148-55. https://doi.org/10.7705/biomedica.v34i0.1646
McKinnon PS, Davis SL. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur J Clin Microbiol Infect Dis. 2004;23:271-88. http://dx.doi.org/10.1007/s10096-004-1107-7
Arnau J, Vallano A. Estudio de utilización de medicamentos. Medicamentos y Salud. 2000;2:78-82.
Elseviers M, Andersen M, Benko R, Bennie M, Godman B, Vander Stichele R, et al. Drug utilization research: Methods and applications. 1st edition. Chichester: John Wiley & Sons; 2016. p. 3-14.
Vaca CP, de las Salas RP, López JJ, Sánchez R, Figueras A. Algorithm for the evaluation of therapeutic failure reportsproposal and pilot analysis. Pharmacoepidemiol Drug Saf. 2013;22:199-206. https://doi.org/10.1002/pds.3355
Saturni S, Bellini F, Braido F, Paggiaro P, Sanduzzi A, Scichilone N, et al. Randomized controlled trials and real life studies. Approaches and methodologies: A clinical point of view. Pulm Pharmacol Ther. 2014;27:129-38. https://doi.org/10.1016/j.pupt.2014.01.005
Angkasekwinai N, Werarak P, Chaiyasoot K, Thamlikitkul V. Monitoring of effectiveness and safety of generic formulation of meropenem for treatment of infections at Siriraj Hospital. J Med Assoc Thai. 2011;94(Suppl.1):S217-24.
Badaró R, Molinar F, Seas C, Stamboulian D, Mendonça J, Massud J, et al. A multicenter comparative study of cefepime versus broad-spectrum antibacterial therapy in moderate and severe bacterial infections. Braz J Infect Dis. 2002;6:206-18. https://doi.org/10.1590/S1413-86702002000500001
Lizarazo M, Andrea N, Villamil L, Alicia M, Lopera-Velásquez V, Robledo J, et al. Characterization of the infection prevention and control programs in hospitals located in Medellín - Colombia, 2011. Infectio. 2013;17:136-45. https://doi.org/10.1016/S0123-9392(13)70720-3
Carrillo P, Álvarez CA, Arboleda D, Yomayusa N. Estado actual de los comités de infecciones en cinco ciudades de Colombia. Rev Med Sanitas. 2010;13:34-9.
Rattanaumpawan P, Sutha P, Thamlikitkul V. Effectiveness of drug use evaluation and antibiotic authorization on patients’ clinical outcomes, antibiotic consumption, and antibiotic expenditures. Am J Infect Control. 2010;38:38-43. https://doi.org/10.1016/j.ajic.2009.04.288
Llor C, Bjerrum L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5:229-41. https://doi.org/10.1177/2042098614554919
Murthy R. Implementation of strategies to control antimicrobial resistance. Chest. 2001;119(Suppl.2):405S-11S. https://doi.org/10.1378/chest.119.2_suppl.405S
Torres NMC, Rodríguez JJ. Notificación de las reacciones adversas a los medicamentos antiepilépticos en Bogotá (Colombia, 2008-2012). Rev Cuba Neurol Neurocir. 2014;4:117-23.
Monterrosa DA, Bolívar HC. Caracterización de las reacciones adversas a medicamentos (RAMs) observadas en una institución asistencial de III nivel de complejidad. Cienc E Innov En Salud. 2015;3:11-16. https://doi.org/10.17081/innosa.3.2.90
Truven Health Analytics. Micromedex. Fecha de consulta: 12 de enero de 2017. Disponible en: www.micromedex. com/support
Figueras A, Pedrós C, Valsecia M, Laporte J-R. Therapeutic ineffectiveness: Heads or tails? Drug Saf. 2002;25:485-7. https://doi.org/10.2165/00002018-200225070-00002
Some similar items:
- Jorge Enrique Machado-Alba, Luis Felipe Calvo-Torres, Andrés Gaviria-Mendoza, Juan Daniel Castrillón-Spitia, Prescribing patterns of antiparkinson drugs in a group of Colombian patients, 2015 , Biomedica: Vol. 38 No. 3 (2018)
- Jorge Machado, John Alexander Alzate, Patterns of antiretroviral drug prescripcion in 997 Colombian patients , Biomedica: Vol. 28 No. 1 (2008)
- Constanza Pardo, Ricardo Cendales, Survival analysis of cervical cancer patients , Biomedica: Vol. 29 No. 3 (2009)
- Raúl Murillo, Ricardo Cendales, Carolina Wiesner, Marion Piñeros, Sandra Tovar, Effectiveness of cytology-based cervical cancer screening in the Colombian health system , Biomedica: Vol. 29 No. 3 (2009)
- Sandra Lorena Girón, Julio César Mateus, Fabián Méndez, Impact of an open waste disposal site on the occurrence of respiratory symptoms and on health care costs of children , Biomedica: Vol. 29 No. 3 (2009)
- José Joaquín Carvajal, Ligia Inés Moncada, Mauricio Humberto Rodríguez, Ligia del Pilar Pérez, Víctor Alberto Olano, Characterization of Aedes albopictus (Skuse, 1894) (Diptera:Culicidae) larval habitats near the Amazon River in Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Andrés Páez, Gloria Rey, Carlos Agudelo, Alvaro Dulce, Edgar Parra, Hernando Díaz-Granados, Damaris Heredia, Luis Polo, Outbreak of urban rabies transmitted by dogs in Santa Marta, northern Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Patricia Escobar, Katherine Paola Luna, Indira Paola Hernández, César Mauricio Rueda, María Magdalena Zorro, Simon L. Croft, In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole , Biomedica: Vol. 29 No. 3 (2009)
- Jaime E. Bernal, Martha Lucía Tamayo , Ignacio Briceño , Escilda Benavides , Newborn screening in Colombia: The experience of a private program in Bogotá , Biomedica: Vol. 44 No. 1 (2024)
- Mauricio Beltrán, María Cristina Navas, María Patricia Arbeláez, Jorge Donado, Sergio Jaramillo, Fernando De la Hoz, Cecilia Estrada, Lucía del Pilar Cortés, Amalia de Maldonado, Gloria Rey, Seroprevalence of hepatitis B virus and human immunodeficiency virus infection in a population of multiply-transfused patients in Colombia , Biomedica: Vol. 29 No. 2 (2009)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























