Laboratory-based surveillance of Shigella spp. from human clinical cases in Colombia, 1997-2018
Abstract
Introduction: Shigellosis is endemic in low-and middle-income countries, causing approximately 125 million episodes of diarrhea and leading to approximately 160 .000 deaths annually one-third of which is associated with children.
Objective: To describe the characteristics and antimicrobial resistance profiles of Shigella species recovered in Colombia from 1997 to 2018.
Materials and methods: We received isolates from laboratories in 29 Colombian departments. We serotyped with specific antiserum and determined antimicrobial resistance and minimal inhibitory concentrations for ten antibiotics with Kirby-Bauer tests following the Clinical and Laboratory Standards Institute recommendations.
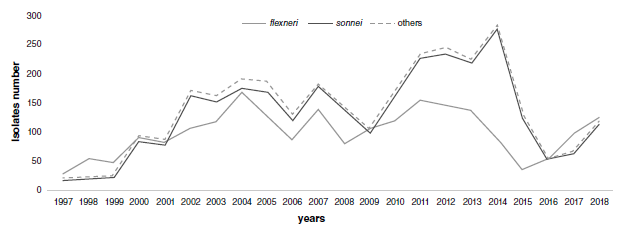
Results: We analyzed 5,251 isolates of Shigella spp., most of them obtained from stools (96.4%); 2,511 (47.8%) were from children under five years of age. The two most common species were S. sonnei (55.1%) and S. flexneri (41.7%). The highest resistance rate was that of tetracycline (88.1%) followed by trimethoprim-sulfamethoxazole (79.3%) and ampicillin (65.5%); 50.8% of isolates were resistant to chloramphenicol, 43.6% to amoxicillin/clavulanic acid, and less than 1% to cefotaxime, ceftazidime, gentamicin, and ciprofloxacin. In S. sonnei, the most common resistance profile corresponded to trimethoprim-sulfamethoxazole (92%) whereas in S. flexneri the most common antibiotic profiles were multidrug resistance.
Conclusions. In Colombia, children under five years are affected by all Shigella species. These findings should guide funders and public health officials to make evidence based decisions for protection and prevention measures. The antimicrobial resistance characteristics found in this study underline the importance of combating the dissemination of the most frequently isolated species, S. sonnei and S. flexneri.
Downloads
References
GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909-48. https://doi.org/10.1016/S1473-3099(17)30276
Bardhan P, Faruque ASG, Naheed A, Sack DA. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis. 2010; 1:1718-23. https://doi.org/ 10.3201/eid1611.090934
Mattock E, Blocker AJ. How do the virulence factors of Shigella work together to cause disease? Front Cell Infect Microbiol. 2017;7:64. https://doi.org/10.3389/fcimb.2017.00064
Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet. 2016;388:1291-301. https://doi.org/ 10.1016/S0140-6736(16)31529-X.
Muthuirulandi Sethuvel DP, Devanga Ragupathi NK, Anandan S, Veeraraghavan B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett Appl Microbiol. 2017;1:8-18. https://doi.org/10.1111/lam.12690
DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126. https://doi.org/10.1093/infdis/159.6.1126
World Health Organization. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. Accessed: May 21, 2019. Available from: https://www.who.int/cholera/publications/shigellosis/en/
Anderson M, Sansonetti PJ, Marteyn BS. Shigella diversity and changing landscape: Insights for the twenty-first century. Front Cell Infect Microbiol. 2016;6:45. https://doi.org/10.3389/fcimb.2016.00045
Chang Z, Lu S, Chen L, Jin Q, Yang J. Causative species and serotypes of shigellosis in mainland China: Systematic review and meta-analysis. PLOS ONE. 2012;7:52515. https://doi.org/10.1371/journal.pone.0052515
Baker S, The HC. Recent insights into Shigella. Curr Opin Infect Dis. 2018;31:449-54. https://doi.org/10.1097/QCO.0000000000000475
Ewing WH. Edwards and Ewing’s identification of Enterobacteriaceae. 4th. edition. New York: Elsevier Science Publishing Co., Inc.; 1986. p. 536.
Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-third informational supplement. Wayne: CLSI; 2017.
Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211-28. https://doi.org/10.1016/S1473-3099(18)30310-4
Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MALED). Lancet Glob Health. 2015;18:1191-210. https://doi.org/10.1016/S2214-109X(15)00151-5
López EL, Prado-Jiménez V, O’Ryan-Gallardo M, Contrini MM. Shigella and shiga toxinproducing Escherichia coli causing bloody diarrhea in Latin America. Infect Dis Clin North Am. 2000;14:41-65. https://doi.org/ 10.1016/S0891-5520(05)70217-8
López-Medina E, Parra B, Dávalos DM, López P, Villamarín E, Peláez M. Acute gastroenteritis in a pediatric population from Cali, Colombia in the post rotavirus vaccine era. Int J Infect Dis. 2018;73: 52-9. https://doi.org/10.1016/j.ijid.2018.06.006
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209-22. https://doi.org/10.1016/S0140-6736(13)60844-2
Vinh H, Nhu NTK, Nga TVT, Duy PT, Campbell JI, Hoang NVM, et al. A changing picture of shigellosis in southern Vietnam: Shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect Dis. 2009;9:204. https://doi.org/10.1186/1471-2334-9-204
Thompson CN, Duy PT, Baker S. The rising dominance of Shigella sonnei: An intercontinental shift in the etiology of bacillary dysentery. PLoS Negl Trop Dis. 2015;9:e0003708. https://doi.org/10.1371/journal.pntd.0003708
Vasco G, Trueba G, Atherton R, Calvopiña M, Cevallos W, Andrade T, et al. Identifying etiological agents causing diarrhea in low income Ecuadorian communities. Am J Trop Med Hyg. 2014; 91:563-9. https://doi.org/10.4269/ajtmh.13-0744
Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;6:614-27. https://doi.org/10.1093/oxfordjournals.aje.a116134
Qiu S, Xu X, Yang C, Wang J, Liang B, Li P, et al. Shift in serotype distribution of Shigella species in China, 2003-2013. Clin Microbiol Infect. 2015;21:252. https://doi.org/10.1016/j.cmi.2014.10.019
Faruque ASG, Ahmed AMS, Ahmed T, Islam MM, Hossain MI, Roy SK, et al. Nutrition: Basis for healthy children and mothers in Bangladesh. J Health Popul Nutr.2008;26:325-39. https://doi.org/10.3329/jhpn.v26i3.1899
Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: A systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42-52. https://doi.org/10.1016/S1473-3099(04)01253-8
Sayeed S, Sack DA, Qadri F. Protection from Shigella sonnei infection by immunisation of rabbits with Plesiomonas shigelloides (SVC O1). J Med Microbiol. 1992;6:382-4. https://doi.org/10.1099/00222615-37-6-382
Esrey SA, Feachem RG, Hughes JM. Interventions for the control of diarrhoeal diseases among young children: Improving water supplies and excreta disposal facilities. Bull World Health Organ. 1985;63:757-72.
Ram PK, Crump JA, Gupta SK, Miller MA, Mintz ED. Part II. Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984-2005. Epidemiol Infect. 2008;136:577-603. https://doi.org/10.1017/S0950268807009351
Löfdahl M, Ivarsson S, Andersson S, Långmark J, Plym-Forshell L. An outbreak of Shigella dysenteriae in Sweden, May-June 2009, with sugar snaps as the suspected source. Eurosurveillance. 2009;14:19268. https://doi.org/10.2807/ese.14.28.19268-en
Smith AM, Keddy KH, Sooka A, Ismail H, DeJong GM. Analysis of a temporal cluster of Shigella boydii isolates in Mpumalanga, South Africa, November to December 2007. J Infect Dev Ctries. 2009;3:65-70. https://doi.org/10.3855/jidc.107
Ud-Din AIMS, Wahid SUH, Latif HA, Shahnaij M, Akter M, Azmi IJ, et al. Changing trends in the prevalence of Shigella species: Emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLOS ONE. 2013;8:e82601. https://doi.org/10.1371/journal.pone.0082601
Zafar A, Hasan R, Nizami SQ, von Seidlein L, Soofi S, Ahsan T, et al. Frequency of isolation of various subtypes and antimicrobial resistance of Shigella from urban slums of Karachi, Pakistan. Int J Infect Dis. 2009;6:668-72. https://doi.org/110.1016/j.ijid.2008.10.005
Isenbarger DW, Hien BT, Ha HT, Ha TT, Bodhidatta L, Pang LW, et al. Prospective study of the incidence of diarrhoea and prevalence of bacterial pathogens in a cohort of Vietnamese children along the Red River. Epidemiol Infect. 2001;2:229-36. https://doi.org/10.1017/s0950268801005933
Roy S, Thanasekaran K, Dutta Roy AR, Sehgal SC. Distribution of Shigella enterotoxin genes and secreted autotransporter toxin gene among diverse species and serotypes of Shigella isolated from Andaman Islands, India. Trop Med Int Health. 2006;11:1694-8. https://doi.org/10.1111/j.1365-3156.2006.01723.x
Kahsay AG, Muthupandian S. A review on sero diversity and antimicrobial resistance patterns of Shigella species in Africa, Asia and South America, 2001-2014. BMC Res Notes. 2016;9:422. https://doi.org/ 10.1186/s13104-016-2236-7
Streit JM, Jones RN, Toleman MA, Stratchounski LS, Fritsche TR. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: Report from the SENTRY Antimicrobial Surveillance Program (2003). Int J Antimicrob Agents. 2006;27:367-75. https://doi.org/10.1016/j.ijantimicag.2005.12.004
Penatti MPA, Hollanda LM, Nakazato G, Campos TA, Lancellotti M, Angellini M, et al. Epidemiological characterization of resistance and PCR typing of Shigella flexneri and Shigella sonnei strains isolated from bacillary dysentery cases in Southeast Brazil. Braz J Med Biol Res. 2007;40:249-58. https://doi.org/10.1590/S0100-879X2006005000069
Seribelli AAp, Frazão MR, Medeiros MIC, Falcão JP. Molecular and phenotypic characterization of strains of Shigella sonnei isolated over 31 years suggests the circulation of two prevalent subtypes in São Paulo State, Brazil. J Med Microbiol. 2016;65:666-77. https://doi.org/10.1099/jmm.0.000290
Khaghani S, Shamsizadeh A, Nikfar R, Hesami A. Shigella flexneri: A three-year antimicrobial resistance monitoring of isolates in a Children Hospital, Ahvaz, Iran. Iran J Microbiol. 2014;6:225-9.
Cui X, Wang J, Yang C, Liang B, Ma Q, Yi S, et al. Prevalence and antimicrobial resistance of Shigella flexneri serotype 2 variant in China. Front Microbiol. 2015;6:435. https://doi.org/10.3389/fmicb.2015.00435
Lima AA, Sidrim JJ, Lima NL, Titlow W, Evans ME, Greenberg RN. Molecular epidemiology of multiply antibiotic-resistant Shigella flexneri in Fortaleza, Brazil. J Clin Microbiol. 1997;35:1061-5. https://doi.org/10.1128/JCM.35.5.1061-1065.1997
Khatun F, Faruque A, Koeck JL, Olliaro P, Millet P, Paria N, et al. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980-2008). Epidemiol Infect. 2011;139:446-52. https://doi.org/10.1017/S0950268810001093
Taneja N. Changing epidemiology of shigellosis and emergence of ciprofloxacin-resistant Shigellae in India. J Clin Microbiol. 2007;45:678-9. https://doi.org/10.1128/JCM.02247-06
Wasfy MO, Oyofo BA, David JC, Ismail TF, el-Gendy AM, Mohran ZS, et al. Isolation and antibiotic susceptibility of Salmonella, Shigella, and Campylobacter from acute enteric infections in Egypt. J Health Popul Nutr. 2000;18:33-8.
El-Gendy A, Mansour A, Weiner M, Pimentel G, Armstrong A, Young S, et al. Genetic diversity and antibiotic resistance in Shigella dysenteriae and Shigella boydii strains isolated from children aged <5 years in Egypt. Epidemiol Infect. 2012;140:299-310. https://doi.org/10.1017/S0950268811000525
von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: Disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3:353. https://doi.org/10.1371/journal.pmed.0030353
Vrints M, Mairiaux E, van Meervenne E, Collard J-M, Bertrand S. Surveillance of antibiotic susceptibility patterns among Shigella sonnei strains isolated in Belgium during the 18-year period 1990 to 2007. J Clin Microbiol. 2009;47:1379-85. https://doi.org/10.1128/JCM.02460-08
Kim J-Y, Kim S-H, Jeon S-M, Park M-S, Rhie H-G, Lee B-K. Resistance to fluoroquinolones by the combination of target site mutations and enhanced expression of genes for efflux pumps in Shigella flexneri and Shigella sonnei strains isolated in Korea. Clin Microbiol Infect. 2008;14:760-5. https://doi.org/10.1111/j.1469-0691.2008.02033.x
Jain SK, Gupta A, Glanz B, Dick J, Siberry GK. Antimicrobial-resistant Shigella sonnei: Limited antimicrobial treatment options for children and challenges of interpreting in vitro azithromycin susceptibility. Pediatr Infect Dis J. 2005;24:494-7. https://doi.org/ 10.1097/01.inf.0000164707.13624.a7
Boumghar-Bourtchai L, Mariani-Kurkdjian P, Bingen E, Filliol I, Dhalluin A, Ifrane SA, et al. Macrolide-resistant Shigella sonnei. Emerg Infect Dis. 2008;14:1297-9. https://doi.org/10.3201/eid1408.080147
Some similar items:
- César A. Arias, Marylin Hidalgo, Jinnethe Reyes, Ana María Cárdenas, Lorena Díaz, Sandra Ríncon, Natasha Vanegas, Paula Lucía Díaz, Elizabeth Castañeda, Resistance profiles to fluoroquinolones in clinical isolates of Gram positive cocci , Biomedica: Vol. 28 No. 2 (2008)
- José Ramón Mantilla, María Teresa Reguero, Elsa Beatriz González, Ibonne Ayde García, Aura Lucía Leal, Paula Andrea Espinal, Celia Alpuche, Ismael Alberto Valderrama, Martha Isabel Garzón, Narda María Olarte, Molecular characterization of an outbreak caused by CTX-M-12-producing Klebsiella pneumoniae in a Colombian hospital´s neonatal intensive care unit , Biomedica: Vol. 26 No. 3 (2006)
- Andrea Patricia Villalobos, Liliana Isabel Barrero, Sandra Milena Rivera, María Victoria Ovalle, Danik Valera, Surveillance of healthcare associated infections, bacterial resistance and antibiotic consumption in high-complexity hospitals in Colombia, 2011 , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Cristina Lucía Mora, Julián Castaño, María Consuelo Jaramillo, Inhibitory activity of dihydroxy-phenyl-propenone on betalactamase of Enterobacter cloacae: Preliminary study for drug development to overcome bacterial resistance , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Jancy Andrea Huertas, Proposal to establish an environmental contaminants surveillance system in Colombia , Biomedica: Vol. 35 (2015): Agosto, Suplemento 2, Salud y contaminantes ambientales
- José Julián López, Yira Cortázar, Ángela Acosta, Claudia Marcela Vargas-Peláez, Francisco Rossi, Drug utilization study of two generic antibiotics in a tertiary hospital in Bogotá , Biomedica: Vol. 38 No. 3 (2018)
- Liliana Hilarión-Gaitán, Diana Díaz-Jiménez, Karol Cotes-Cantillo, Carlos Castañeda-Orjuela, Inequalities in health by regime of affiliation to the health system in events of obligatory notification, Colombia, 2015 , Biomedica: Vol. 39 No. 4 (2019)
- Wilmer Giovanny Mosquera, Libeth Yajaira Criado , Beatriz Elena Guerra, Antimicrobial activity of endophytic fungi from the medicinal plants Mammea americana (Calophyllaceae) and Moringa oleifera (Moringaceae) , Biomedica: Vol. 40 No. 1 (2020)
- Manuel Enrique Machado-Duque, Katherine Mercado-Gómez , María Camila Bernal-Chica , Stephanie Uribe-Vélez , Jorge Enrique Machado-Alba, Prescription and indications for the use of fluoroquinolones in a group of outpatients in Colombia , Biomedica: Vol. 40 No. 2 (2020)
- Daniela Salas, Dora Yurany Sánchez, Germán Achury, Fabio Escobar-Díaz, Malaria in populations with mining occupation, Colombia, 2012-2018 , Biomedica: Vol. 41 No. Supl. 1 (2021): Mayo, Parasitología médica
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























